Table of Contents
Overview
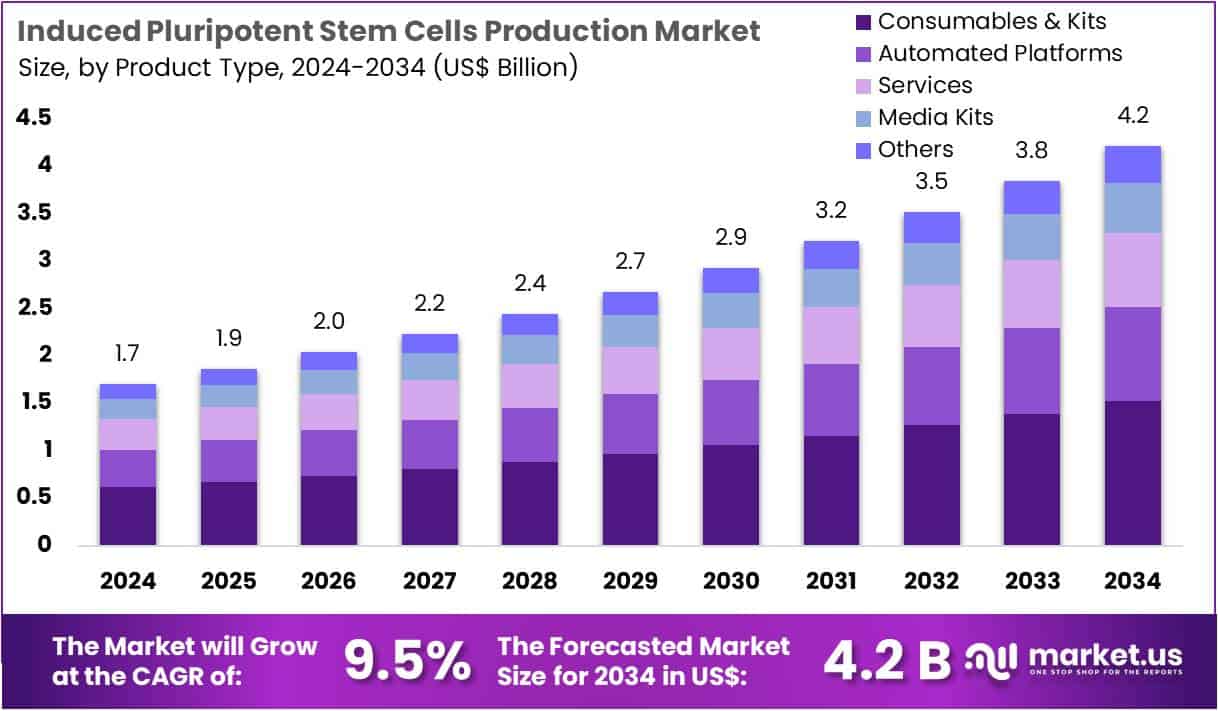
New York, NY – Aug 11, 2025: The Global Induced Pluripotent Stem Cells (iPSC) Production Market is projected to grow from USD 1.7 Billion in 2024 to USD 4.2 Billion by 2034, expanding at a CAGR of 9.5% between 2025 and 2034. iPSCs are adult cells reprogrammed to behave like stem cells. These cells are widely used in regenerative medicine to potentially treat diseases affecting the brain, heart, liver, and more. At the core of this process are growth factors, which are proteins that direct how cells grow and function.
Growth factors play a vital role throughout the iPSC production process. In the early stages, they help reprogram adult cells back into a pluripotent state. After reprogramming, they help maintain these cells in their undifferentiated form. Later, specific growth factors are used to guide the cells into becoming desired tissue types. This step-by-step control is necessary to ensure accurate, safe, and reliable results for therapeutic use or research.
Key types of growth factors include fibroblast growth factors (FGFs) and transforming growth factors (TGFs). FGFs are important for keeping the iPSCs stable and healthy in laboratory conditions. TGFs help preserve stemness while also assisting in the early stages of differentiation. Other essential proteins include epidermal growth factor (EGF) and insulin-like growth factor (IGF), which support cell survival and improve overall efficiency in the reprogramming process.
To convert iPSCs into specific types of cells, scientists use targeted growth factors. For example, brain-derived neurotrophic factors (BDNFs) help generate neurons, while vascular endothelial growth factors (VEGFs) aid in developing heart and vascular cells. Each tissue type requires a precise combination of signals. This ensures the cells differentiate correctly, reducing risks such as incomplete transformation or contamination.
Global health agencies such as the U.S. FDA and the European Medicines Agency recommend the use of clinical-grade growth factors. These proteins are produced under strict manufacturing conditions and are free from animal-based ingredients. This improves the safety, purity, and consistency of iPSC-derived therapies. As demand for cell-based treatments rises, maintaining high standards in growth factor quality will be crucial for regulatory approval and clinical success.
Key Takeaways
- In 2024, the Induced pluripotent stem cells production market generated US$ 1.7 billion and is projected to reach US$ 4.2 billion by 2034.
- This market is growing at a compound annual growth rate (CAGR) of 9.5% over the forecast period from 2025 to 2034.
- The product type segment is led by consumables and kits, which captured a 36.2% market share in the year 2024.
- Despite increasing automation, manual methods still dominate the iPSC production landscape due to their widespread use and flexibility.
- In terms of application, drug development and discovery held the top position, contributing 43.6% of the total market revenue.
- The workflow segment includes reprogramming, cell analysis, cell culture, and engineering, with cell culture leading at 33.1% share.
- By end user, biotechnology and pharmaceutical companies held the largest market share at 58.3%, outpacing hospitals and research institutes.
- Regionally, North America led the global market, capturing a 41.3% market share in 2024, driven by strong R&D and infrastructure.
Segmentation Analysis
- Product Type Analysis: Consumables and kits are projected to lead the iPSC production market, capturing 36.2% of the total share. This growth is fueled by the increasing need for high-quality tools that support reliable and repeatable cell production. As the use of iPSCs grows in fields like drug discovery, regenerative medicine, and toxicity screening, demand for efficient kits is rising. These products offer ease of use and consistency, making them indispensable in both research and commercial settings. Enhanced kit formulations are also improving control and output, further boosting this segment’s expansion.
- Process Analysis: Manual iPSC production remains the most widely used process, holding a market share of 61.8%. This method is preferred due to its flexibility and lower initial costs compared to automated systems. It allows researchers to fine-tune protocols based on specific needs, particularly in regenerative research. While automation is gaining ground, many laboratories still value the hands-on control that manual processes provide. This approach offers adaptability that is difficult to match, especially when precision is required to produce targeted cell types for research or clinical use.
- Application Analysis: The drug development and discovery segment dominates iPSC applications, accounting for 43.6% of the market. iPSCs are valuable in building accurate disease models, which improve the quality of preclinical testing. These cells reflect human biology more closely than traditional models, enhancing early drug screening. Pharmaceutical and biotech companies are increasingly adopting iPSC technologies to support innovation. As the demand for human-relevant, predictive models rises, iPSCs are becoming vital tools in the process of developing safer and more effective therapies.
- Workflow Analysis: Cell culture is the most critical workflow in iPSC production, representing 33.1% of the market. This step is essential for enabling cell growth and transformation into the desired types. The segment benefits from advances in culture media and protocols that enhance reproducibility and efficiency. Improved conditions help reduce cell variability and increase yields, making the process more reliable. These developments are key to meeting the growing demand for quality-controlled iPSC lines used in drug testing, regenerative therapy, and disease research.
- End-User Analysis: Biotechnology and pharmaceutical companies dominate the end-user segment, holding 58.3% of the iPSC production market. Their deep investment in iPSC research supports breakthroughs in drug development, personalized medicine, and regenerative therapies. iPSCs offer advanced capabilities for modeling diseases and screening new drugs. These companies often collaborate with suppliers to improve production processes and accelerate innovation. With continued focus on cell-based and tailored treatments, demand for robust and scalable iPSC systems is expected to increase significantly across commercial sectors.
Regional Analysis
North America Leads the Induced Pluripotent Stem Cells Production Market
North America accounted for the highest revenue share of 41.3% in the iPSC production market. This leadership is driven by strong government investments and a growing pipeline of clinical trials. The U.S. National Institutes of Health (NIH) allocated approximately US$48.6 billion in 2024, with a significant portion directed toward stem cell research. ClinicalTrials.gov data shows an increasing number of iPSC-based studies in the U.S. Companies like Thermo Fisher Scientific, with US$13.43 billion in 2023 revenue, supply vital tools supporting this expanding demand.
Asia Pacific Set to Record the Fastest Growth in iPSC Production
Asia Pacific is projected to witness the highest CAGR during the forecast period. This rapid growth is fueled by increased government support and a surge in clinical research activities. Japan’s AMED allocated roughly US$1.35 billion in 2024 for medical R&D, directly aiding iPSC advancements. The number of iPSC-related trials is rising in countries such as Japan and China. As a result, demand for scalable and high-quality cell production services is increasing. Industry leaders like Thermo Fisher are strengthening their regional presence to meet this growing market need.
Key Players Analysis
Leading companies in the induced pluripotent stem cell (iPSC) production market are adopting key strategies to accelerate growth. They are expanding their product offerings by developing advanced iPSC lines and improving differentiation protocols to support various research and clinical needs. To enhance scalability and consistency, many firms are adopting automation and high-throughput technologies. Collaborations with academic institutions and biotech companies are fostering innovation. In addition, companies are setting up manufacturing units and distribution networks in strategic locations to meet the rising demand for iPSC-based products.
FUJIFILM Cellular Dynamics, Inc., headquartered in Madison, Wisconsin, is a major player in the iPSC production space. The company manufactures human iPSC-derived cells for use in drug discovery, toxicology screening, and regenerative medicine. Its flagship products, including iCell and MyCell lines, are widely adopted by pharmaceutical companies and research organizations. FUJIFILM Cellular Dynamics prioritizes quality and reproducibility in its cell models. Backed by FUJIFILM Holdings, it utilizes cutting-edge technologies and global infrastructure to advance iPSC-based innovations in healthcare.
Emerging Trends
- Shift Toward Chemical Reprogramming: Researchers are working on safer ways to create Induced pluripotent stem cells productions. One major change is using only small chemical molecules. This means they avoid adding outside genes to reprogram cells. It makes the process cleaner and easier to control. Chemical methods also reduce risks linked to gene-based tools. This trend is gaining attention, especially for therapies where gene-free techniques are safer. It could open new doors for clinical use. The shift also helps in making more stable and predictable cell lines. As the technology improves, chemical reprogramming may become the standard for iPSC production.
- Better Quality Control with Single-Cell Analysis: Single-cell analysis is changing how scientists check Induced pluripotent stem cells production quality. This method looks at each cell one by one. It helps find problems early in the production process. In the past, testing only checked groups of cells, so hidden issues could be missed. Now, scientists can see how individual cells behave and develop. This improves safety and reliability. It also helps in creating better cell lines for therapy or research. As the tools become cheaper and faster, single-cell analysis is likely to become a standard step in iPSC quality control.
- Use of iPSCs in Space and Microgravity: iPSCs are now being tested in space. Scientists are studying how they grow in microgravity. Early results show that stem cells may form better tissues without gravity. This could help make stronger organ models. These tests are done on space stations by research teams and space agencies. The goal is to improve health care in space and on Earth. Growing tissues in space might lead to new ways to build organs for transplants. This trend also supports long-term space missions where medical help is limited. It’s a bold step into future medicine.
- Rise of Complex Organoids and Tissue Models: iPSCs are now used to create organoids—tiny, 3D versions of human organs. These models can look and act like real organs, such as the brain, heart, or liver. They are used to study how diseases start and grow. Scientists also use them to test how drugs affect human cells. This reduces the need for animal testing. Organoids help researchers see how tissues form and change. They are also used in personalized medicine, using a patient’s own cells. As this field grows, lab-grown mini-organs may become a key tool in medical research.
Use Cases
- Studying Diseases in the Lab: Induced pluripotent stem cells (iPSCs) are made from a person’s own cells. Scientists can turn them into brain, liver, or heart cells. These lab-made cells help researchers study how diseases like Alzheimer’s, Parkinson’s, and heart disease begin and develop. This process allows them to test new treatments in a human-like model without using animals. It also shows how different patients may react to the same illness. This makes treatments more targeted and effective. iPSCs help speed up discovery while being more ethical than traditional methods. Overall, they offer a powerful tool for studying diseases at a deeper level.
- Testing Drugs More Safely: Drug companies use Induced pluripotent stem cells production -derived human cells to test new medicines. These cells act like real human tissues, offering a more accurate view of how a drug will work. Unlike animal testing, iPSCs can reflect human responses better. They also help detect harmful side effects early in the research phase. This lowers risks before clinical trials begin. iPSC testing can also save money and time by screening many compounds at once. As a result, drug development becomes safer, quicker, and more effective. It leads to smarter decision-making and better outcomes for patients and researchers alike.
- Personalized Medicine: Because iPSCs come from the patient’s own cells, they can be used to create custom treatments. This reduces the risk of rejection and improves success rates. Scientists can also fix faulty genes in these cells using gene editing tools like CRISPR. This makes the cells safer and more effective. iPSCs allow doctors to study how a patient’s specific genes affect their health. This helps them tailor therapies for each person. It’s especially useful for rare or inherited diseases. Personalized iPSC medicine is a major step toward precision healthcare that fits the unique needs of every patient.
- Tissue Engineering and Lab-Grown Organs: Scientists are using iPSCs to build small tissues in the lab that function like real organs. These include mini-versions of the brain, heart, and liver, also called organoids. They help doctors study disease and test drugs without using animals or human donors. In the future, researchers hope to grow full-sized organs using iPSCs. These lab-grown organs could replace damaged ones, solving the problem of organ shortages. iPSC-based tissues are also useful in surgeries and transplant research. They may soon play a major role in personalized organ repair and long-term healthcare solutions.
Conclusion
The induced pluripotent stem cell (iPSC) production market is expanding rapidly, driven by its powerful role in research, drug testing, and regenerative medicine. iPSCs offer a safer, more personalized approach to healthcare by using a patient’s own cells to create targeted treatments. Growth factors and advanced technologies like automation, organoids, and gene editing are improving both quality and outcomes.
Key players are investing in innovation and global infrastructure to meet rising demand. As more countries support clinical research and safer production practices, iPSCs are set to become a standard tool in modern medicine. Their potential to transform diagnostics and therapies makes them a critical part of the future healthcare landscape.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



