Overview
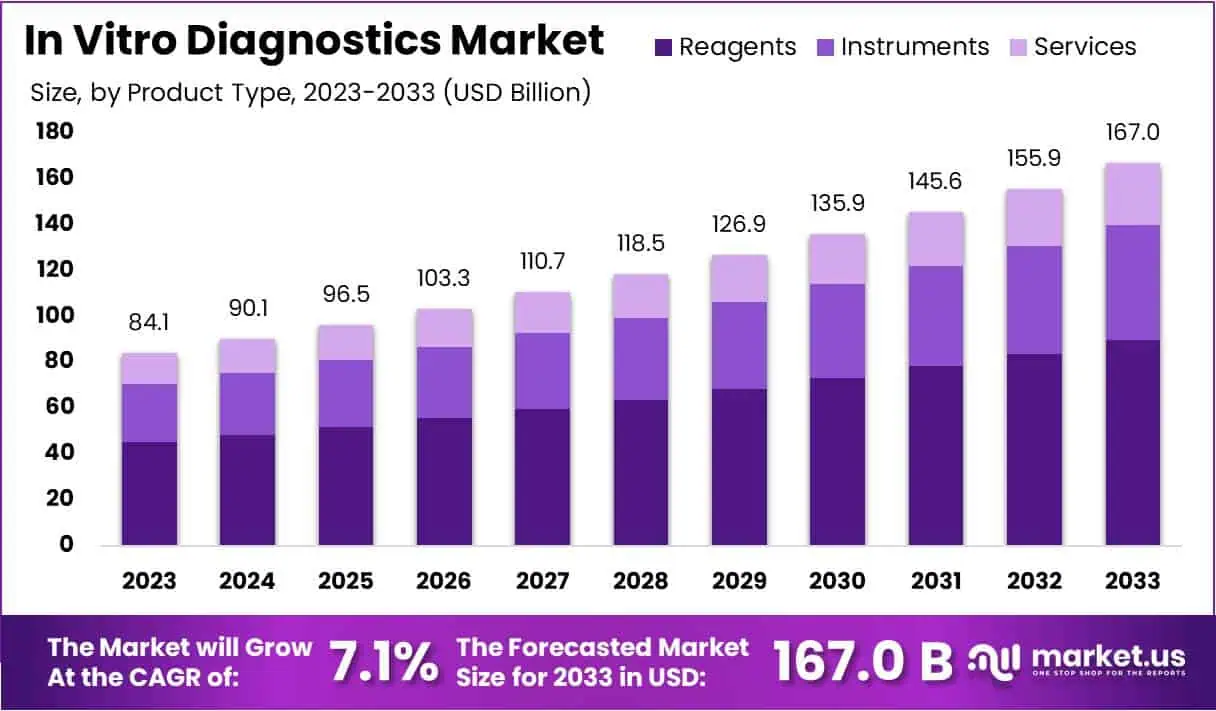
New York, NY – August 28, 2025: The In Vitro Diagnostics (IVD) Market is projected to reach US$ 167.0 billion by 2033, growing from US$ 84.1 billion in 2023 at a CAGR of 7.1%. A key factor driving this growth is the rising burden of chronic diseases worldwide. According to WHO, the number of people living with diabetes increased from 200 million in 1990 to 830 million in 2022. The International Diabetes Federation adds that cases may rise to 853 million by 2050, highlighting strong demand for diagnostic solutions.
The ageing global population further accelerates the demand for IVD products. United Nations projections indicate that the number of people aged 60 years and above will reach 1.4 billion by 2030, rising from 1.1 billion in 2023. By 2050, this figure will double to 2.1 billion. Older populations typically require frequent testing for cardiovascular diseases, cancers, and metabolic conditions. This demographic shift will result in increased utilization of diagnostic technologies, thereby creating long-term opportunities for market players worldwide.
The increasing prevalence of noncommunicable diseases (NCDs) also drives the IVD market significantly. WHO reports that NCDs such as cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes account for nearly 75% of global deaths. These diseases place heavy pressure on healthcare systems, making early diagnosis and monitoring critical. IVD tools play an essential role in timely detection, disease management, and treatment evaluation. As the global burden of NCDs rises, healthcare providers are expected to depend more on accurate and affordable diagnostic solutions.
In addition, disparities in access to healthcare and diagnostic services in low- and middle-income countries present both challenges and opportunities. Although a majority of diabetes and other NCD patients live in these regions, treatment and diagnostic access remain limited. This unmet need creates demand for affordable and scalable diagnostic solutions. Companies investing in accessible technologies will benefit from strong adoption. Addressing healthcare inequality is not only a public health priority but also a potential market expansion driver for IVD manufacturers.
Supportive policies and regulatory emphasis on diagnostics are strengthening the sector. WHO’s Essential Diagnostics List underscores the critical role of diagnostic testing in improving public health outcomes. National programs such as India’s NPCDCS have integrated screening and monitoring into primary healthcare systems, reflecting strong governmental commitment. These initiatives promote wider adoption of diagnostic tests, encourage infrastructure development, and increase investment in research. Policy support is expected to continue reinforcing the importance of diagnostics as a cornerstone of effective disease prevention and management strategies.
Key Takeaways
- In 2023, the In Vitro Diagnostics (IVD) market generated US$ 84.1 billion revenue and is projected to reach US$ 167.0 billion by 2033.
- The market demonstrated a compound annual growth rate (CAGR) of 7.1%, indicating steady expansion and increasing adoption of diagnostic technologies worldwide.
- Product segmentation shows reagents, instruments, and services, with reagents dominating in 2023, accounting for 53.8% of the overall market share.
- Based on technology, molecular diagnostics led with a 39.5% market share, surpassing hematology, immunology, coagulation, clinical chemistry, microbiology, and other diagnostic technologies.
- Within applications, infectious diseases accounted for the highest share of 44.3%, ahead of diabetes, oncology, cardiology, nephrology, drug testing, and autoimmune diseases.
- Regarding end-use, hospitals contributed 61.9% of revenues, positioning themselves as the leading adopters compared to laboratories and other smaller healthcare settings.
- Regionally, North America led the global IVD market in 2023, securing 39.2% market share, driven by advanced infrastructure and high diagnostic demand.
Regional Analysis
North America leads the in vitro diagnostics (IVD) market, accounting for a 39.2% revenue share. This dominance is supported by the rising incidence of chronic diseases and the strong emphasis on preventive healthcare. Demand for advanced diagnostic tools enabling early disease detection has increased significantly. Furthermore, technological advancements in laboratory automation and point-of-care testing have improved diagnostic speed and accuracy. These factors have created a favorable environment, positioning North America as the strongest region in the global in vitro diagnostics industry.
A notable milestone in this region occurred in July 2023, when Siemens secured FDA clearance for its Atellica CI Analyzer. This system is designed for immunoassay and clinical chemistry applications, offering reliable and timely test results. Its introduction into global markets reflects the growing adoption of innovative diagnostic solutions. Such developments emphasize the integration of advanced technologies in laboratories across North America. These advancements continue to strengthen the region’s competitive edge and contribute to sustained market growth.
In contrast, the Asia Pacific region is projected to record the highest CAGR during the forecast period. The growth outlook is driven by the increasing prevalence of chronic and infectious diseases, alongside a rapidly aging population. Countries such as India, China, and Japan are investing heavily in healthcare infrastructure and diagnostic technology. This expansion is improving access to innovative diagnostic tools and services. As a result, the Asia Pacific market is positioned to achieve strong and sustained growth in the coming years.
Recent product launches further highlight this regional momentum. In January 2023, FUJIFILM Sonosite introduced the Sonosite PX ultrasound system in India, aiming to enhance clinical efficiency and ergonomics. Such initiatives underline the commitment of Asia Pacific countries toward modernizing healthcare delivery. Rising awareness of early disease detection and the increasing acceptance of home-based testing are also contributing to market expansion. Collectively, these drivers indicate that Asia Pacific will emerge as the fastest-growing in vitro diagnostics market globally.
Emerging Trends
- Rise of Point-of-Care Testing (POCT): Point-of-Care Testing (POCT) is becoming a game-changer in the IVD sector. Tests are moving beyond central labs and reaching clinics, pharmacies, and even homes. Rapid kits for diabetes, infectious diseases, and COVID-19 are now widely used. Patients get faster results without waiting for lab reports. This reduces hospital visits and improves access to care. POCT also helps in early treatment decisions, which improves outcomes. The convenience and speed of POCT are pushing healthcare toward a more patient-friendly model. This trend will continue to grow as technology becomes simpler and more affordable.
- Digital and AI Integration: Artificial Intelligence and digital health tools are transforming IVD. Devices are now integrated with AI-based platforms that analyze results faster and more accurately. These tools also allow remote monitoring and predictive diagnosis. For example, AI can detect patterns in test results that humans may miss. Patients benefit from quicker insights and more personalized care. Digital platforms also make it easy to share results with doctors instantly. This improves decision-making and reduces delays in treatment. The use of AI in IVD is expected to grow as healthcare providers demand smarter, connected, and more efficient solutions.
- Growth of Molecular Diagnostics: Molecular diagnostics is seeing strong demand in the IVD market. Techniques like PCR and genetic testing are being used more widely. They are especially important in cancer detection, infectious disease management, and early diagnosis. By identifying genetic mutations, doctors can choose treatments that work best for each patient. This shift is also driving research in precision medicine. Molecular tests are known for their accuracy and speed. They are playing a major role in reducing misdiagnosis and improving patient outcomes. The demand for molecular diagnostics will rise further as costs fall and awareness grows among patients and doctors.
- Home-Based Testing Expansion: Home-based testing is gaining popularity worldwide. People now use self-test kits for pregnancy, cholesterol, and infectious diseases. These kits bring healthcare directly into homes, making it more accessible. Patients can monitor their health without frequent clinic visits. This reduces pressure on hospitals and empowers individuals to take charge of their health. Home-based testing also saves time and lowers costs. The demand grew rapidly during COVID-19 and continues to expand. Convenience, privacy, and faster decision-making are the key drivers. As technology improves, more reliable and user-friendly self-testing kits are expected to enter the market.
- Focus on Personalized Medicine: Personalized medicine is reshaping how diseases are treated. IVD plays a key role by analyzing genetic and molecular markers. This helps doctors design treatments based on individual patient needs. Instead of a “one-size-fits-all” approach, patients get therapies tailored to their unique profile. Personalized testing is especially useful in cancer and rare diseases. It improves treatment effectiveness and reduces side effects. Patients receive better outcomes while healthcare providers reduce wasted costs. With advancements in IVD, the future of personalized medicine looks stronger. This approach is set to become standard in modern healthcare systems worldwide.
- Increased Regulatory and Quality Standards: Regulations around IVD are getting stricter worldwide. Governments and health agencies are raising quality standards to ensure tests are accurate and reliable. While this improves patient trust, it also challenges manufacturers to maintain compliance. Companies are now focusing on innovation while meeting tough regulatory checks. This ensures that patients receive safe and dependable diagnostic solutions. Stricter rules also create fair competition in the market. In the long run, better quality standards will drive industry growth. High trust in test accuracy will encourage more adoption and strengthen the role of IVD in modern healthcare.
Use Cases
- Infectious Disease Testing: In vitro diagnostics play a vital role in identifying infectious diseases such as HIV, hepatitis, tuberculosis, influenza, and COVID-19. These tests are fast and accurate, which makes them highly valuable in hospitals, labs, and clinics. Early detection allows doctors to start treatment on time and control the spread of infections in communities. Rapid testing methods, including PCR and antigen tests, have become especially important during outbreaks. They help health professionals act quickly, prevent large-scale transmission, and save lives. As a result, infectious disease testing remains one of the most crucial applications of IVD worldwide.
- Cancer Detection and Monitoring: IVD has transformed the way cancer is detected and managed. Molecular and genetic tests can identify cancer in its early stages, even before symptoms appear. These tests provide insights into tumor biology, which helps doctors recommend the most effective therapies. IVD is also used to track how well a treatment is working and to detect if cancer comes back. This continuous monitoring gives patients a better chance of successful outcomes. With personalized medicine growing, cancer-related IVD tests are becoming more advanced, reliable, and essential in oncology care around the globe.
- Diabetes Management: Diabetes care is one of the most common uses of in vitro diagnostics. Millions of patients rely on blood glucose testing kits to check sugar levels at home or in clinics. These tests are quick, affordable, and easy to use, making them an everyday tool for patients and doctors. Continuous glucose monitoring devices, powered by IVD technology, provide real-time insights. They help in adjusting diet, exercise, and medications effectively. Without IVD tests, managing diabetes would be far more challenging. They ensure patients can track their condition closely and maintain healthier lifestyles with timely interventions.
- Cardiovascular Disease Risk Assessment: Cardiovascular diseases remain a leading cause of death worldwide. IVD tests help assess the risk by analyzing cholesterol, lipid profiles, and cardiac biomarkers. These blood-based tests detect early signs of heart issues, allowing preventive care before conditions worsen. For example, troponin tests are used in emergency cases to confirm heart attacks quickly. Regular screenings also help doctors track long-term heart health in patients with risk factors. With IVD, healthcare providers can personalize treatment, prevent complications, and promote better lifestyle decisions. This makes cardiovascular testing a vital tool in reducing heart-related mortality globally.
- Prenatal and Genetic Testing: Expecting mothers and families benefit greatly from prenatal and genetic IVD testing. Non-invasive prenatal testing (NIPT) allows the detection of genetic abnormalities early in pregnancy. This helps parents and doctors prepare for possible complications and plan care effectively. Genetic screening can also reveal inherited disorders, guiding families in making informed decisions. These tests are safe, reliable, and widely adopted in maternity care. With IVD technology, early detection improves outcomes for both mother and baby. As awareness grows, prenatal and genetic testing is becoming a routine part of modern healthcare practices worldwide.
- Chronic Disease Monitoring: Patients with long-term conditions such as kidney disease, liver disorders, or autoimmune diseases rely on IVD for continuous monitoring. Routine blood and urine tests give doctors clear insights into organ function and treatment response. These tests ensure that therapies are working and allow early detection of complications. For example, creatinine levels are measured to track kidney health, while liver function tests help monitor damage caused by chronic conditions. Regular IVD testing empowers patients to manage their illnesses better. It also allows doctors to adjust treatments promptly, improving long-term outcomes and overall quality of life.
- Blood Banking and Transfusion Safety: Safe blood supply is critical in healthcare, and IVD plays a major role in ensuring it. Blood donations are carefully tested to screen for infections such as HIV, hepatitis, and syphilis. Compatibility tests are also performed to match donor blood with recipients, reducing the risk of transfusion reactions. These procedures safeguard patients during surgeries, emergencies, and treatments requiring transfusion. Without IVD, the risk of transmitting diseases through blood would be far higher. Reliable diagnostics make blood banking safer, efficient, and trustworthy. They ensure that patients receive only safe, compatible, and infection-free blood products.
Conclusion
The in vitro diagnostics market is set for strong and steady growth, supported by rising cases of chronic and infectious diseases, an ageing global population, and the need for early and accurate testing. Advances in molecular diagnostics, digital health, and home-based testing are reshaping the way healthcare providers and patients approach disease management. Growing demand in both developed and emerging regions highlights the importance of affordable and accessible solutions. With supportive government policies and continuous innovation, IVD is becoming a central pillar of modern healthcare. The future outlook shows that diagnostics will play an even greater role in improving outcomes and reducing the global health burden.
View More
Point-of-Care Molecular Diagnostics Market || PCR Molecular Diagnostics Market || Core Clinical Molecular Diagnostics Market || Companion Diagnostics Market || Liquid Biopsy Market || Self Testing Market || At-Home Testing Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



