Table of Contents
Introduction
The global In Vitro Diagnostics (IVD) market is set to grow from USD 95.3 billion in 2022 to USD 149.4 billion by 2032, at a CAGR of 4.7%. Key growth drivers include the rising demand for personalized medicine, where IVD tests are crucial. These tests help tailor treatments to individual patients by identifying genetic variations that influence drug responses. This capability enhances treatment outcomes, making personalized medicine an integral component of modern healthcare. Next-generation sequencing tests are especially important in this context, as they offer precise genetic analysis.
Technological advancements are significantly boosting the IVD market. Innovations such as point-of-care and home testing kits have made diagnostics more accessible and convenient. These technologies enable quicker diagnosis and treatment, which is particularly beneficial in remote or resource-limited areas. As a result, the use of IVDs is expanding beyond traditional laboratories, providing a broader reach and facilitating timely medical interventions. This trend is vital in ensuring healthcare delivery is efficient and effective across various settings.
Regulatory support also plays a crucial role in the growth of the IVD market. Agencies like the FDA have streamlined approval processes for IVD devices, especially during public health emergencies. The introduction of Emergency Use Authorizations (EUAs) during the COVID-19 pandemic allowed for rapid test deployment. Such regulatory flexibility highlights the importance of a supportive framework in fostering market growth. These measures have significantly contributed to the timely availability of essential diagnostic tools, crucial in managing health crises.
The global focus on disease prevention and management has further increased the adoption of IVDs. These diagnostics are vital in the early detection of infectious diseases, enabling timely intervention and reducing healthcare costs. The World Health Organization emphasizes the importance of IVDs in achieving universal health coverage by ensuring access to essential diagnostic services. This global emphasis on healthcare accessibility and disease management is driving market expansion, with IVDs playing a central role in modern healthcare strategies.
Recent developments in the IVD market include significant innovations and strategic partnerships. Thermo Fisher Scientific and Sysmex Corporation are leading in these advancements. In November 2022, Thermo Fisher Scientific launched the Accula Flu A/Flu B test, a rapid RT-PCR test that differentiates influenza A and B in about 30 minutes. In October 2022, Thermo Fisher acquired The Binding Site Group to enhance its capabilities in specialty diagnostics. Meanwhile, Sysmex introduced the UF-1500 Fully Automated Urine Particle Analyzer, reflecting the increasing demand for automated and precise diagnostic instruments.
Key Takeaways
- Market Growth: The Global In Vitro Diagnostics (IVD) Market is growing at a compound annual growth rate (CAGR) of 4.7%.
- Technology Advancements: Automated IVD systems, like molecular diagnostics, enable swift, accurate diagnoses with innovations such as ultrasensitive molecular tests and point-of-care testing.
- Product and Services: IVD tests various bodily fluids and tissues using reagents, software, and instruments, including molecular diagnostics and immunodiagnostics.
- End-Users: Main users of IVD products include laboratories, hospitals, and point-of-care facilities, with laboratories having the largest market share.
- Applications: Infectious diseases are the leading application segment, diagnosing microorganisms like pneumonia, hepatitis, and HIV/AIDS. Chronic diseases also drive market growth.
- Drivers: The increasing incidence of chronic diseases and the growing elderly population drive demand for regular checkups, boosting the IVD market.
- Restraints: The high cost of IVD instruments and the need for skilled professionals, along with unclear reimbursement policies, pose challenges.
- Trends: Technological advancements in diagnostic tools and a preference for real-time data from point-of-care devices are key trends.
- Opportunities: Emerging markets like South Korea, India, and China offer growth opportunities due to adaptable regulations and rising healthcare investments.
- COVID-19 Impact: The pandemic increased IVD usage for COVID-19 diagnostics, but non-essential testing decreased, affecting overall market dynamics.
- Regional Analysis: North America leads the market, contributing over 38% of revenue in 2022, while Asia Pacific is the fastest-growing region.
- Key Players: Major players include Siemens Healthcare, Bio-Rad Laboratories, Abbott Diagnostics, and Qiagen N.V., focusing on product expansion and innovation.
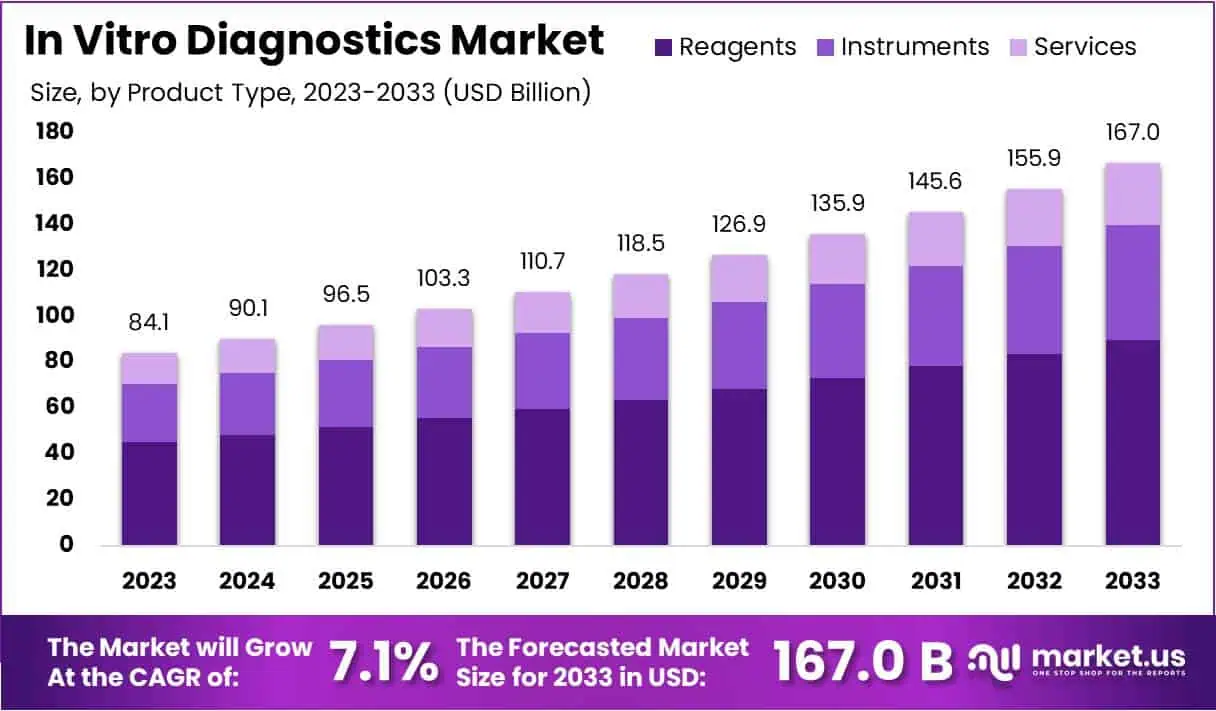
In Vitro Diagnostics (IVD) Statistics
- 2022: The market began at $95.3 billion, with reagents being the top contributor.
- 2023: It rose to $99.8 billion, showing steady growth.
- 2024: Increased further to $103.9 billion as technology advanced.
- 2025: Reached $110.0 billion, continuing its upward trend.
- 2026: Expanded to $115.9 billion, reflecting sustained industry interest.
- 2027: Grew to $120.6 billion, supported by innovations in diagnostics.
- 2028: Climbed to $124.1 billion, as demand for testing increased.
- 2029: Surged to $129.9 billion, driven by global health needs.
- 2030: Achieved $135.3 billion, marking significant market expansion.
- 2031: Increased to $141.6 billion, nearing future projections.
- 2032: Expected to hit $149.4 billion, culminating a decade of growth.
- Approximately 3.3 billion IVD tests are performed annually, although specific data on lab-developed tests (LDTs) are not comprehensively tracked.
- HCE on IVDs in the US and Germany: Accounts for 2.3% and 1.4% of total healthcare expenditure, respectively.
- Physicians’ Perception of IVD Spending: 81% believed IVD costs were more than 5% of total healthcare costs, which is incorrect.
- Correct Estimation by Physicians: Only 19% of physicians accurately estimated IVD spending as between 0–4% of total HCE.
- Perception of Spending Appropriateness: After learning actual costs, 64% of physicians felt the spending on IVD was appropriate.
- Decision-Making Based on IVD: 66% of clinical decisions were influenced by IVD results.
- Physicians’ Demand for Data: 53% of physicians want IVD tests to show clinical evidence of improving patient outcomes.
- Interest in Health Economic Benefits: 29% of physicians believe IVDs should provide both clinical benefits and health economic advantages.
- Physicians Asking for More Data: 83% of physicians requested more clinical or combined clinical/health economic data than the product alone.
- The FDA regulates IVDs under a risk-based classification system, where tests are categorized as Class I (low risk), Class II (moderate risk), and Class III (high risk), with most high-risk tests requiring premarket approval.
- The Pew Charitable Trusts highlights that IVDs play a crucial role in precision medicine, particularly in genetic and molecular profiling.
- Synthetic nucleic acids are increasingly used in developing rapid diagnostic tools for controlling viral diseases, addressing challenges in culturing certain viruses.
- Certified reference materials, like DNA from breast cancer and synthetic SARS-CoV-2 RNA, are pivotal for challenging the performance of assays during validation processes.
Emerging Trends
- Integration of Artificial Intelligence (AI) in Diagnostics: Artificial Intelligence (AI) is transforming in vitro diagnostics by improving the analysis and interpretation of data. This advancement enables quicker and more precise diagnostic outcomes, which are critical for early detection of diseases and the development of personalized treatment plans. AI’s role is particularly significant in complex areas such as oncology and genetics, where its ability to process vast amounts of data can lead to better diagnostic accuracy and efficiency. As technology advances, AI-powered diagnostics are expected to become increasingly common in healthcare settings, enhancing both the speed and accuracy of medical diagnostics.
- Advancements in Point-of-Care Testing: The demand for point-of-care (POC) testing is on the rise, enabling healthcare providers to conduct diagnostic tests promptly at or near the patient care site. This trend is propelled by the necessity for immediate results, crucial in emergency and critical care scenarios. POC testing is particularly beneficial in rural and underserved regions, where traditional lab facilities may be scarce. These devices offer rapid diagnostics, supporting timely medical decisions and improving patient outcomes in various healthcare settings.
- Growth of Home-Based Testing: Home-based diagnostic tests have surged in popularity, driven by their convenience and the ease with which they can be used. The COVID-19 pandemic played a significant role in accelerating the adoption of home testing kits, not only for detecting the virus but also for other infectious diseases. Looking forward, the trend towards home testing is expected to expand, covering a broader spectrum of conditions. This shift promises to make routine diagnostics more accessible to the public, encouraging proactive health management from the comfort of home.
- Personalized Medicine and Companion Diagnostics: Personalized medicine is increasingly influencing the use of in vitro diagnostics, particularly through companion diagnostics. These tests help customize treatment plans based on an individual’s genetic profile, ensuring more effective therapy, especially in oncology. The ability to tailor treatments to specific genetic markers of diseases like cancer is enhancing therapeutic outcomes and optimizing treatment protocols, marking a significant shift towards more personalized healthcare solutions.
- Automation and Digitalization in Diagnostic Laboratories: The automation of laboratory processes is gaining traction, enhancing the efficiency and accuracy of diagnostic tests. Automated systems minimize human error and boost processing capacity, addressing the growing demand for diagnostic services. Additionally, the digitalization of lab results improves data management and integrates seamlessly with electronic health records. This evolution in laboratory technology not only speeds up the diagnostic process but also ensures greater reliability and consistency in test results.
Use Cases
- Cancer Diagnostics: In vitro diagnostics (IVD) are pivotal in diagnosing and managing cancer. They leverage tests like liquid biopsies and genomic assays to detect cancer biomarkers, essential for tailoring personalized treatment strategies. In 2023, the oncology segment of IVD experienced robust growth, propelled by breakthroughs in molecular diagnostics. These advancements enhance the accuracy of cancer detection and the efficacy of subsequent treatments, underscoring the critical role of IVD in oncology.
- Infectious Disease Testing: IVD is indispensable in identifying infectious diseases, including COVID-19, influenza, and tuberculosis. Techniques such as rapid antigen and molecular tests deliver prompt and precise results. These are crucial for effective disease management and containment strategies. The significance of IVD was particularly underscored during the COVID-19 pandemic, proving vital in global health initiatives and response efforts.
- Chronic Disease Monitoring: IVD tests are routinely employed to monitor chronic diseases, aiding in the management of conditions like diabetes and cardiovascular diseases. Tests such as the HbA1c for diabetes and lipid panels for assessing cardiovascular risk are commonly utilized. These tests provide ongoing insights into patient health, enabling better disease management and preventive care strategies.
- Genetic and Prenatal Testing: IVD also extends to genetic applications, including newborn screening, carrier testing, and non-invasive prenatal testing (NIPT). These tests yield essential data regarding genetic disorders and potential health risks, facilitating informed healthcare decisions by both patients and providers. The availability of these tests enhances preventative care and supports early intervention strategies.
- Neurological Testing: Innovations in IVD have introduced diagnostic tests for neurological disorders, such as Alzheimer’s disease and traumatic brain injuries. These tests, which identify specific biomarkers, offer prospects for early diagnosis. Early detection can greatly influence treatment approaches and improve patient outcomes, highlighting the growing importance of IVD in neurology.
Conclusion
The In Vitro Diagnostics (IVD) market is poised for substantial growth, fueled by the demand for personalized medicine and advancements in diagnostic technologies. As these tests become more integral to customizing patient care, the market’s expansion is further supported by regulatory enhancements and a global emphasis on preventive healthcare. Innovations such as point-of-care and home testing kits are making diagnostics more accessible, enhancing patient outcomes, particularly in underserved areas. Additionally, the strategic developments by leading players like Thermo Fisher Scientific and Sysmex Corporation signify robust industry dynamics. Moving forward, the integration of artificial intelligence and the shift towards home-based testing are expected to drive further growth, revolutionizing how healthcare providers diagnose and manage diseases.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



