Introduction
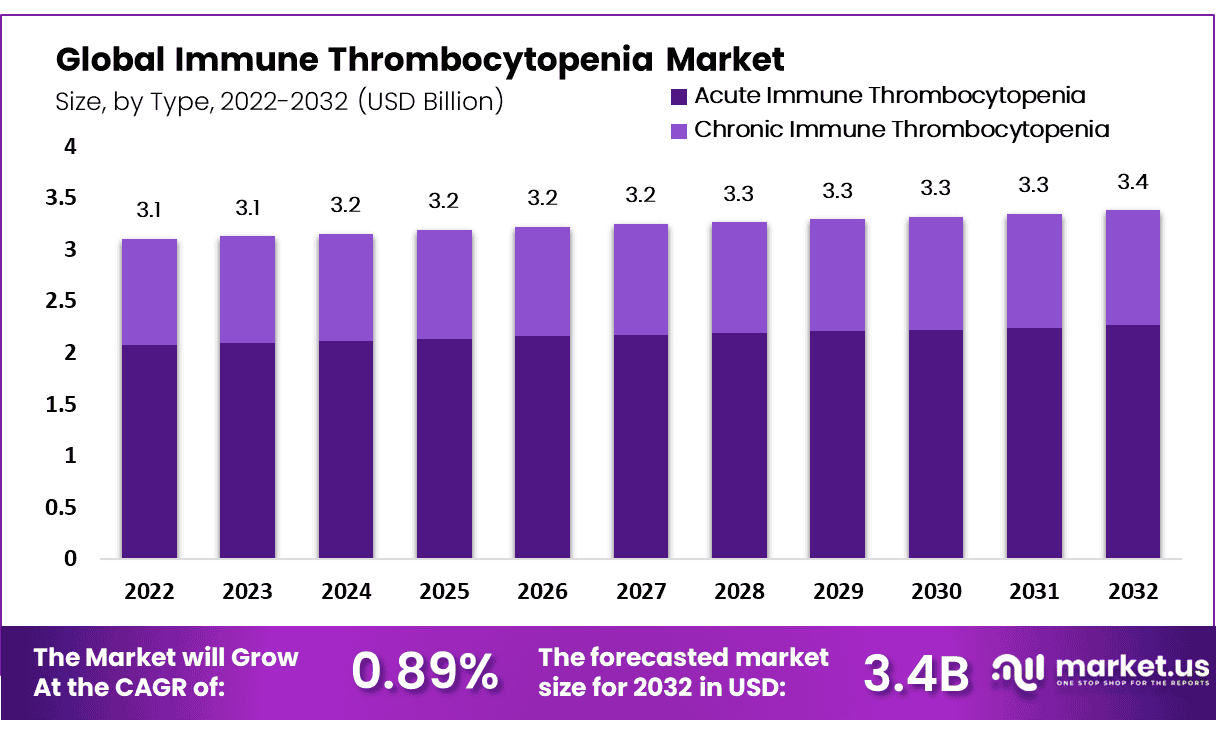
The Immune Thrombocytopenia Market, projected to grow from USD 3.1 billion in 2022 to USD 3.4 billion by 2032, reflects a modest CAGR of 0.89%. This growth is supported by multifaceted factors including clinical insights, demographic trends, advancements in treatments, and the contributions of health organizations backed by government data.
In the U.S., an analysis of treatment patterns among ITP patients highlights significant efficacy gaps, particularly for those with chronic conditions. Many remain unresponsive to conventional treatments like corticosteroids and immunoglobulins. This situation points to the chronic nature of ITP and the variability in patient responses, emphasizing the need for innovative therapeutic options.
Despite the availability of treatments, challenges such as treatment-related fatigue and bleeding concerns markedly affect the quality of life for patients. Studies suggest that improvements in platelet counts do not necessarily correlate with relief from symptoms like fatigue. This discrepancy indicates a need for treatments that not only manage platelet counts but also address symptom control, advocating for a more personalized approach to ITP management.
Government and health organizations are crucial in understanding and managing ITP. Insights from national health databases and patient registries provide valuable information on the epidemiology and treatment outcomes, guiding research and policy decisions. These resources aid in tracking disease incidence, treatment patterns, and healthcare utilization, which are integral to refining treatment protocols and enhancing patient outcomes.
The Immune Thrombocytopenia Market’s potential growth is driven by a deeper understanding of the disease, the development of new therapies, and substantial support from healthcare systems and government initiatives focused on research and patient support. Continued collaboration among clinical researchers, healthcare providers, and government health organizations is vital to meeting the complex needs of ITP patients and improving their life quality.
Key Takeaways
- The market is set to grow to USD 3.4 billion by 2032, advancing at a modest 0.89% CAGR from 2023 to 2032.
- A global increase in ITP cases across all age groups is pushing demand for effective treatments.
- Enhanced diagnostic tools are improving detection accuracy, leading to better early management and market growth.
- Rising awareness among both patients and healthcare professionals is leading to more frequent diagnoses.
- New treatment innovations are propelling market expansion and improving outcomes for patients.
- Challenges like underdiagnosis due to awareness gaps, treatment costs, and side effects hinder market accessibility.
- There’s growing potential for targeted therapies that precisely address immune dysregulation associated with ITP.
- Telemedicine and digital health tools are revolutionizing patient engagement, allowing for efficient, remote management of ITP.
- North America holds the largest market share at 48%, driven by its advanced healthcare infrastructure and active research environment.
- Asia-Pacific is the fastest-growing region, spurred by large patient populations and increasing healthcare investments.
Regional Analysis
North America holds the dominant position in the global immune thrombocytopenia (ITP) market, commanding a 48% share in 2022. The region benefits from an established healthcare infrastructure, heightened awareness, and state-of-the-art diagnostic and treatment options. These factors contribute significantly to its leading market stance.
The area is home to many prominent pharmaceutical companies and research institutions dedicated to ITP research and development. North America’s robust presence in the sector underscores its pivotal role in driving innovations and advancements in ITP treatment.
Asia-Pacific is projected to be the fastest-growing region in the immune thrombocytopenia market during the forecast period. This growth is primarily driven by a large patient base, enhancements in healthcare infrastructure, and increasing awareness of the condition.
Significant contributions from emerging economies like China and India accelerate the regional market’s expansion. The growing presence of pharmaceutical manufacturers and escalating healthcare investments are further propelling the market growth in Asia-Pacific.
Emerging Trends
- Bruton Tyrosine Kinase (BTK) Inhibitors: The use of Rilzabrutinib, a BTK inhibitor, has demonstrated encouraging outcomes in phase I/II trials. It achieved a 40% response rate, indicating its effectiveness in rapidly and durably increasing platelet counts. Importantly, this treatment does so without causing severe side effects, making it a potentially valuable option for patients needing sustainable recovery.
- Neonatal Fc Receptor (FcRn) Inhibitors: Efgartigimod works by targeting the FcRn to decrease immunoglobulin levels. In clinical trials, this drug showed a sustained response in 22% of patients, compared to only 5% in those receiving a placebo. This significant difference highlights its potential as a viable treatment for managing ITP.
- Complement Inhibitors: Another promising therapy is Sutimlimab, which targets the classical complement pathway. In a phase I study, 42% of patients with chronic refractory ITP responded to the treatment. This response rate underscores the potential of complement inhibitors in treating this challenging condition.
- Anti-CD38 Therapy: Daratumumab is currently under investigation for its potential role in depleting long-lived plasma cells. These cells are responsible for producing antiplatelet autoantibodies, and their depletion could be crucial in treating ITP effectively.
Use Cases
- Thrombopoietin Receptor Agonists (TPO-RAs): TPO-RAs serve as the standard second-line treatments for adult Immune Thrombocytopenia (ITP). Medications like eltrombopag and romiplostim are particularly notable. Eltrombopag, for example, has proven highly effective. In clinical studies, it has achieved durable responses in 58% of patients who had undergone a splenectomy, and 66% in those who had not. This indicates its significant role in managing the condition effectively over time.
- Rituximab: This therapy is a cornerstone for treating refractory adult ITP. Rituximab has been successful in inducing long-term responses, with effectiveness ranging from 18% to 35% among patients. Its use underscores the importance of having robust options available for those who do not respond to initial treatments, offering hope and improved health outcomes.
- Personalized Treatment Approaches: There is an increasing focus on personalized medicine within ITP management. Early diagnosis and customized treatment plans are pivotal. The use of predictive biomarkers, such as thrombopoietin levels, is becoming more common. This approach helps in selecting the most effective therapy tailored to individual patient needs, thereby enhancing overall treatment success and patient satisfaction.
Conclusion
The Immune Thrombocytopenia market, with its projected growth driven by advances in clinical research and supportive health policies, highlights a burgeoning need for tailored treatment options. The increasing prevalence of ITP across all demographics necessitates innovations that address both platelet management and symptomatic care to enhance patient quality of life. Despite the availability of several treatment modalities, gaps in treatment efficacy and the chronic nature of the disease underscore the ongoing demand for more effective solutions. The market’s expansion is poised to continue, supported by advancements in therapeutic options and enhanced diagnostic techniques, fostering a more personalized and effective approach to managing this complex condition.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



