Table of Contents
Introduction
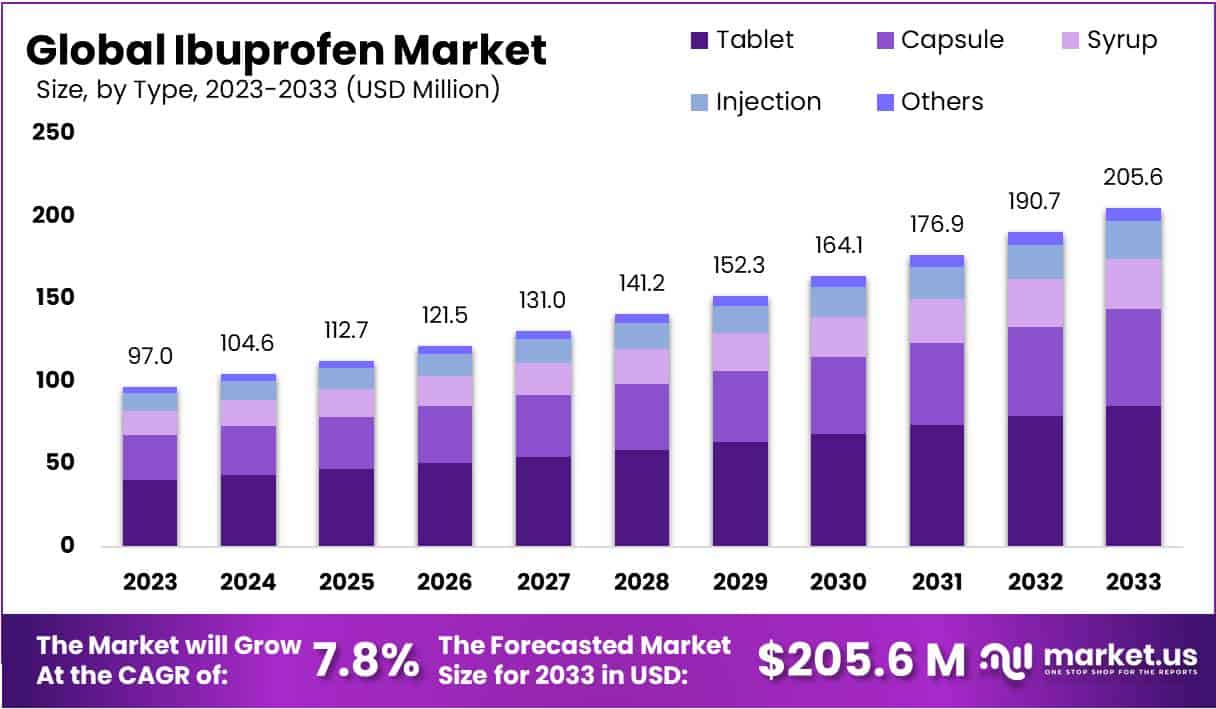
The Ibuprofen market is poised for significant growth, projected to nearly double from a 2023 valuation of USD 97 million to approximately USD 205.6 million by 2033, advancing at a Compound Annual Growth Rate (CAGR) of 7.8%. This growth is propelled by the expanding over-the-counter (OTC) market, as consumers increasingly opt for self-medication, creating opportunities for manufacturers to boost OTC Ibuprofen sales.
However, the market faces challenges, notably from stringent regulatory standards and competition from alternative pain management therapies. These factors require companies to innovate continuously and navigate complex approval processes for new products. Additionally, the trend towards eco-friendly packaging and combination therapies poses both a challenge and an opportunity, as companies need to balance consumer demands for sustainability with the clinical effectiveness of their products.
Recent developments in the market include strategic collaborations aimed at enhancing product offerings and expanding market reach. Noteworthy examples from 2023 include the inauguration of a new manufacturing facility by Solara Active Pharma Sciences, and a partnership between BASF and Recipharm to develop innovative Ibuprofen formulations. These initiatives are indicative of the dynamic efforts within the industry to meet growing market demands and adapt to evolving consumer preferences.
Key Takeaways
- Market Size and Growth: The Ibuprofen market is projected to reach USD 205.6 million by 2033, growing at a CAGR of 7.8% from USD 97 million in 2023.
- Product Preference: In 2023, tablets dominated the market, holding a 41.6% share due to their convenience, with capsules and syrups also playing significant roles.
- Leading Applications: The largest application segments in 2023 were Rheumatoid Arthritis and Osteoarthritis, claiming a 30.8% market share, ahead of cancer-related uses and general pain relief.
- Product Diversity: The market includes various forms like tablets, capsules, syrups, injections, and more, catering to different consumer needs.
- Growth Drivers: Increasing prevalence of chronic pain and an aging population are key factors driving Ibuprofen demand.
- Regulatory Impact: Regulatory challenges and strict approval processes may restrict new Ibuprofen products from entering the market.
- Market Competition: Generic brands and alternative therapies intensify competition, leading to price reductions and narrower profit margins.
- Expansion Opportunities: Opportunities for growth are substantial in emerging markets and through innovations in drug delivery and OTC sales.
- Innovative Trends: The market is witnessing a shift towards combination therapies, sustainable packaging, digital marketing, and personalized medicine.
- Regional Insights: North America held a dominant 41.6% market share in 2023, supported by advanced healthcare systems and a focus on effective pain management.
Ibuprofen Statistics
- Ibuprofen is available in tablets with strengths ranging from 200 to 800 mg.
- Recommended dosages vary from 400 to 800 mg, taken three times daily.
- The drug’s pKa, a measure of acidity, is 5.3, indicating it is almost insoluble in water.
- Peak serum concentrations of ibuprofen occur within 1 to 2 hours post-oral administration.
- Ibuprofen has a short serum half-life of approximately 1.8 to 2 hours.
- Complete elimination of the drug occurs within 24 hours after the last dose.
- The drug binds to proteins at a rate exceeding 99%.
- Extensively metabolized in the liver, only a small fraction of ibuprofen is excreted unchanged.
- Ibuprofen at a dose of 2400 mg/day can completely resolve gouty arthritis symptoms within 72 hours.
- In daily doses of about 2400 mg, ibuprofen’s anti-inflammatory effects are comparable to 4g of aspirin.
- Higher ibuprofen doses, ranging from 1200 to 1600 mg/day, are as effective and well-tolerated as other NSAIDs.
- Osteoarthritis treatment commonly includes NSAIDs, with ibuprofen being a prominent choice.
- Approximately 1% of rheumatoid arthritis patients on NSAIDs may develop major gastrointestinal bleeds.
- Gastric toxicity occurs in 10-32% of patients taking ibuprofen.
- Toxic effects of ibuprofen are unlikely at doses below 100 mg/kg.
- Toxic effects become severe or life-threatening at doses above 400 mg/kg.
- A 400 mg dose of ibuprofen is effective for dental pain control post-third molar surgery.
- Ibuprofen 400 mg in liquid gel form offers faster relief in post-surgical dental pain.
- Over-the-counter ibuprofen is used for minor aches, fever reduction, and dysmenorrhea symptom relief.
- Ibuprofen significantly outperforms placebo in relieving menstrual pain and suppressing menstrual fluid PGF2 alpha.
- Ibuprofen and paracetamol combined can rapidly reduce fever.
- In falciparum malaria, ibuprofen effectively lowers temperatures within the first 4-5 hours.
- In a study, ibuprofen provided a 69% pain relief rate in children and adolescents with migraine.
- Breast cancer reduction, Regular ibuprofen use decreased breast cancer rates by 50%, and aspirin by 40%, indicating potential chemopreventive effects.
- Adverse reactions of NSAIDs, Serious gastrointestinal tract reactions from ibuprofen observed in 1.5% of users, compared to 1% for placebo and 12.5% for aspirin.
Emerging Trends
- Safe Use Guidelines for Ibuprofen: Health authorities have established specific guidelines for the safe consumption of ibuprofen to mitigate adverse effects. The recommended dosage for adults ranges between 400 to 600 mg, up to four times daily, with a maximum limit of 3200 mg per day. For pediatric patients, the dosage is usually calculated based on body weight, with precise measurements provided by healthcare professionals. These measures are crucial to ensure both efficacy and safety in pain management.
- Innovations in Ibuprofen Formulations: Recent advancements in the pharmaceutical industry have led to the development of new ibuprofen formulations. These include slow-release tablets and alternative forms such as gels and sprays, designed to enhance patient adherence and extend the duration of pain relief. Such innovations are particularly beneficial for individuals managing chronic pain, as they provide more consistent symptom control.
- Guidelines for Using Ibuprofen: Medical experts advise taking ibuprofen with food or milk to minimize the risk of stomach upset. Additionally, the use of ibuprofen is generally discouraged beyond the 20th week of pregnancy due to the increased risk of complications to the fetus. Adhering to these recommendations can significantly reduce the potential side effects associated with ibuprofen usage.
- Alternatives to Ibuprofen and Associated Risks: Due to the risks linked with prolonged use of ibuprofen, such as gastrointestinal and cardiovascular issues, health professionals often recommend exploring alternative pain relief methods. This may include the use of other non-steroidal anti-inflammatory drugs (NSAIDs) under specific conditions. Such alternatives can provide effective pain relief while potentially reducing the likelihood of adverse effects.
Use Cases
- Management of Pain and Inflammation: Ibuprofen is frequently chosen for its efficacy in reducing inflammation and alleviating pain linked to various conditions, including arthritis and menstrual cramps. It is particularly valued for its dual ability to address both acute and chronic pain effectively. This makes ibuprofen a versatile and essential tool in pain management protocols, offering relief in a range of clinical scenarios.
- Fever Reduction in Young Populations: Ibuprofen is often the medication of choice for lowering fever in children and adolescents. Its safety profile is preferable to aspirin, which is associated with risks like Reye’s syndrome. By effectively reducing fever without these serious side effects, ibuprofen provides a reliable option for parents and healthcare providers managing pediatric illnesses.
- Application in Special Populations: In individuals suffering from conditions that lead to enhanced inflammation, such as rheumatoid arthritis, ibuprofen is indispensable for pain management. For such populations, healthcare providers carefully calibrate the dosage to optimize therapeutic outcomes while minimizing potential adverse effects. This targeted approach ensures that those most in need receive the benefits of ibuprofen’s pain-relieving properties without undue risk.
Key Players Analysis
- BASF SE, a global leader in chemical production, has significantly invested in enhancing its ibuprofen manufacturing capabilities. The company operates an advanced production facility in Bishop, Texas, which uses a sustainable, award-winning process that minimizes waste and environmental impact. This facility is recognized for its high-quality pharmaceutical ingredients and consistent production output. Additionally, BASF is expanding its ibuprofen production with a new plant in Ludwigshafen, Germany, set to be the first world-scale ibuprofen plant in Europe. This expansion aims to meet growing global demand and ensure reliable supply chains for their customers
- BIOCAUSE Inc. is positioned as a notable player in the global ibuprofen market, focusing on strengthening its market share through strategic partnerships and production enhancements. As part of the industry’s competitive landscape, BIOCAUSE aims to optimize its supply chain to better meet the increasing demand for ibuprofen, a key nonsteroidal anti-inflammatory drug widely utilized for pain relief.
- SI Group Inc., a significant player in the ibuprofen market, has been actively expanding its production capacity to meet the increasing demand driven by an aging population and the growing need for pain management solutions. The company recently boosted production by 10% and announced further expansion plans at its facility in Orangeburg, South Carolina, indicating a proactive approach to addressing market needs. This expansion aims to prevent a supply deficit predicted for the near future due to rising demand, with SI Group being among the top suppliers holding a major share of the industry’s capacity. These strategic moves underscore SI Group’s commitment to maintaining a strong position in the ibuprofen market, which is expected to see a steady growth rate of 2.4% through 2031.
- Pfizer Inc. is actively involved in the Ibuprofen sector, primarily through research aimed at evaluating the cardiovascular and gastrointestinal safety of its products compared to other NSAIDs, such as ibuprofen. Notably, the PRECISION trial, funded by Pfizer and independently directed by the Cleveland Clinic, was a significant study that compared the effects of Pfizer’s Celebrex (celecoxib) with prescription doses of ibuprofen and naproxen in patients with osteoarthritis or rheumatoid arthritis who were at high cardiovascular risk. This study demonstrated that Celebrex had a comparable rate of cardiovascular events to ibuprofen, challenging previous assumptions about the relative safety of these medications. Furthermore, Celebrex was associated with fewer serious gastrointestinal events compared to ibuprofen, highlighting its potential as a safer alternative for long-term use in managing chronic arthritic conditions.
- Abbott Laboratories Ltd. is a significant player in the ibuprofen market, known for its contributions to the development and distribution of this essential nonsteroidal anti-inflammatory drug (NSAID). Abbott, along with other key industry players, is focusing on enhancing product offerings through strategic initiatives such as combining ibuprofen with other active ingredients to increase efficacy and cater to specific patient needs. This approach not only broadens their market reach but also aligns with trends towards personalized medicine, underscoring Abbott’s role in addressing evolving healthcare demands.
- Solara Active Pharma Sciences Ltd., an India-based company recognized for its prowess in the manufacture of Active Pharmaceutical Ingredients (API), has recently expanded its capabilities in the Ibuprofen sector. The company secured a ‘Certificate of Suitability’ (CEP) from the European Directorate for the Quality of Medicines for its state-of-the-art facility in Vishakhapatnam, marking a significant international regulatory endorsement. This approval underscores Solara’s adherence to stringent quality standards and enhances its global footprint in the API market, particularly in the anti-inflammatory segment with Ibuprofen Sodium Dihydrate as one of its key products.
- Shandong Xinhua Pharmaceutical Co. Ltd., established in 1943, is a prominent player in the pharmaceutical sector, notably in the production of ibuprofen. This Chinese firm has made significant strides in the global market, particularly with a strategic move in 2018, acquiring three ibuprofen Abbreviated New Drug Applications (ANDAs) from AiPing Pharmaceutical, which expanded its reach in the U.S. market. Shandong Xinhua is recognized for its comprehensive range of products including not only ibuprofen but also other pharmaceuticals like aspirin and hydrocortisone. Their strategy emphasizes both domestic sales and the expansion into international markets such as the Americas and Europe.
- IOL Chemicals and Pharmaceuticals Ltd. has positioned itself as a global leader in the ibuprofen market, commanding a substantial 35% share. This dominance is underpinned by their integrated manufacturing capabilities, which enhance operational efficiency and product quality. The company’s strategic focus on expanding its ibuprofen segment is supported by continuous investment in research and development, ensuring they stay at the forefront of innovation in this space. These efforts are part of IOL’s broader commitment to specialty chemicals and pharmaceuticals, where they strive to meet global demand effectively and sustainably.
Conclusion
The Ibuprofen market is experiencing substantial growth due to rising consumer preference for over-the-counter pain relief options. This trend is driven by an aging population and increasing instances of chronic pain. Despite challenges such as stringent regulations and competition from alternative therapies, the market is adapting through continuous innovation and strategic collaborations. Recent developments like new manufacturing facilities and partnerships indicate a dynamic industry committed to meeting growing demand and evolving consumer preferences. Companies are also focusing on sustainable packaging and advanced formulations to enhance market appeal. Overall, the Ibuprofen market is poised for continued expansion, with significant opportunities in emerging markets and through product diversification.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



