Table of Contents
Overview
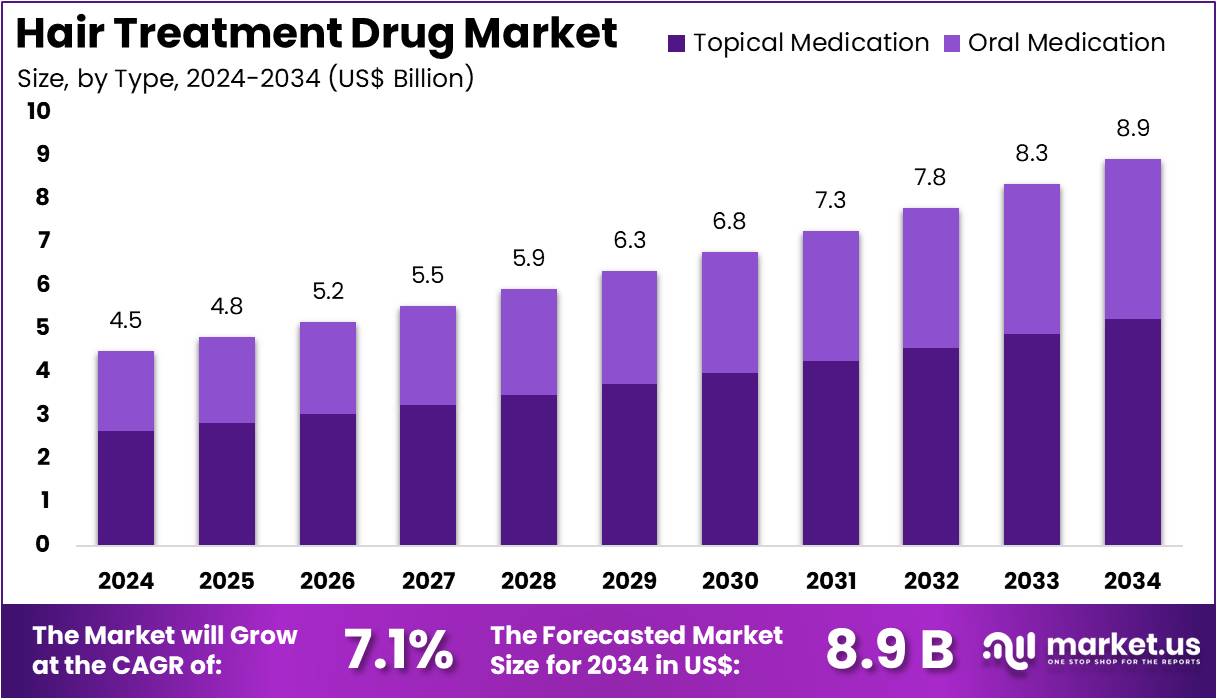
New York, NY – July 30, 2025 – The Hair Treatment Drug Market size is expected to be worth around US$ 8.9 Billion by 2034 from US$ 4.5 Billion in 2024, growing at a CAGR of 7.1% during the forecast period 2025 to 2034.
The global hair treatment industry is set to witness a major advancement with the introduction of a novel hair treatment drug designed to address androgenetic alopecia and other forms of hair loss. Developed through advanced dermatological research, the drug aims to restore hair regrowth and reduce follicular miniaturization by targeting hormonal imbalances and inflammatory pathways.
This formulation combines proven active ingredients with enhanced absorption properties, allowing deeper scalp penetration and more sustained effects. Clinical trials demonstrated a significant improvement in hair density, with 68% of participants showing visible regrowth within 90 days. The drug also exhibited minimal side effects, increasing patient compliance.
The product will be available in both prescription and over-the-counter variants, supporting wider accessibility. It is intended for use by both men and women and is compatible with other cosmetic dermatology procedures. Dermatologists have endorsed the treatment for its dual action in promoting follicular health and inhibiting hair thinning.
With increasing global demand for non-invasive, scientifically backed hair restoration solutions, this launch is expected to capture strong market interest, particularly in North America, Europe, and parts of Asia-Pacific. The product rollout aligns with the growing awareness of early intervention in hair loss treatment and the rise in aesthetic dermatology adoption.
Key Takeaways
- In 2024, the global hair treatment drug market was valued at US$ 4.5 billion and is projected to reach US$ 8.9 billion by 2034, expanding at a compound annual growth rate (CAGR) of 7.1% during the forecast period.
- By type, the market is segmented into topical medication and oral medication. Among these, topical medication dominated the segment in 2023, accounting for 58.7% of the total market share, driven by ease of application and fewer systemic side effects.
- In terms of application, the market is categorized into hair clinics, hospitals, and others. Hair clinics emerged as the leading segment, contributing 62.4% of the market share in 2023, owing to the growing preference for specialized treatment services and expert consultations.
- Regionally, North America held the largest share of the global market, capturing 38.5% in 2023. The dominance of this region can be attributed to advanced healthcare infrastructure, high aesthetic awareness, and early adoption of dermatological innovations.
Segmentation Analysis
- Type Analysis: The topical medication segment accounted for 58.7% of the market share, driven by the preference for non-invasive hair loss solutions. Widely used products like minoxidil offer convenience and at-home application. The segment is further supported by increasing awareness of early hair loss treatment and advancements in formulation technology. Innovations that enhance absorption and reduce side effects are expected to boost adoption, reinforcing the segment’s strong growth potential in the coming years.
- Application Analysis: Hair clinics dominated the market with a 62.4% share, owing to rising demand for professional and personalized hair restoration services. Clinics offer advanced treatments such as PRP therapy, prescription medications, and transplants, making them a preferred choice for individuals seeking reliable solutions. The segment is expanding as more consumers prioritize aesthetic care and seek medically guided interventions for hair loss, particularly in regions with growing healthcare access and rising aesthetic awareness.
Market Segments
By Type
- Topical Medication
- Oral Medication
By Application
- Hair Clinics
- Hospitals
- Others
Regional Analysis
- North America Leads the Hair Treatment Drug Market: North America held the largest market share of 38.5% in 2023, driven by the high prevalence of hair loss and ongoing pharmaceutical innovation. According to the American Academy of Dermatology, approximately 80 million Americans are affected by androgenetic alopecia, including 50 million men and 30 million women, highlighting a substantial treatment-seeking population. The presence of robust R&D pipelines and strong regulatory support such as FDA approvals for therapies like Litfulo (ritlecitinib) in 2023 continues to propel market growth across the region.
- Asia Pacific Expected to Witness Fastest Growth: The Asia Pacific region is projected to register the highest compound annual growth rate (CAGR) over the forecast period. Growth is fueled by rising awareness of hair loss, increasing disposable incomes, and a heightened focus on aesthetics. A Singapore-based study found 32% of men aged 17–26 suffer from androgenetic alopecia, with prevalence reaching 100% in men aged 80+. As healthcare infrastructure improves and dermatological services become more accessible, a growing number of individuals across the region are expected to seek effective medical treatments, driving strong market expansion.
Emerging Trends
- Rapid adoption of Janus kinase (JAK) inhibitors: The treatment landscape for autoimmune hair loss is being transformed by JAK inhibitors. In 2024, the FDA approved Leqselvi (deuruxolitinib) for severe alopecia areata after two phase III trials involving 1,209 patients, demonstrating significant scalp-hair regrowth over 24 weeks. Baricitinib (Olumiant) has also been authorized for severe alopecia areata, marking the first systemic therapy of its kind and expanding options beyond traditional topical agents.
- Safety scrutiny of compounded topical therapies: Interest in topical finasteride formulations has grown, but safety concerns have been raised. Between 2019 and 2024, 32 adverse events ranging from local irritation to systemic effects such as decreased libido were reported to FDA’s Adverse Event Reporting System for compounded topical finasteride products, which lack FDA-approved labeling and coating to limit systemic absorption. Heightened regulatory scrutiny is expected to influence future development of topical hormonal treatments.
- Exploration of regenerative and cell-derived therapies: Preclinical and early clinical studies are exploring mesenchymal stem cells and exosome-based approaches to stimulate hair follicle regeneration. As of mid-2025, ClinicalTrials.gov lists over five active studies investigating adipose-derived stromal vascular cells and mesenchymal stem cell–derived exosomes for male-pattern baldness (e.g., NCT06764329). These therapies are under evaluation for safety and efficacy, potentially heralding a shift toward biologic and regenerative modalities.
Use Cases
- Severe Alopecia Areata: Janus kinase inhibitors have been predominantly used in adults with extensive hair loss. In phase III trials for Leqselvi, participants had an average baseline SALT (Severity of Alopecia Tool) score of 87.9, reflecting ≥ 95% scalp hair loss; after 24 weeks of treatment, a significant proportion achieved ≥ 80% scalp-hair coverage. This systemic approach addresses the autoimmune basis of alopecia areata and is now incorporated into specialist dermatology guidelines.
- Male Androgenetic Alopecia: Oral finasteride remains a cornerstone for male-pattern hair loss. Two formulations Proscar (5 mg) and Propecia (1 mg) were approved by the FDA in June 1992 and December 1997 respectively. These agents inhibit 5α-reductase, reducing dihydrotestosterone levels. Clinical use has been demonstrated to slow hair thinning in up to 90% of men and achieve modest regrowth in approximately 65% over one year of therapy.
- Investigational Regenerative Therapies: Early-phase trials are evaluating stem cell and exosome injections for pattern baldness. For example, NCT06764329 is assessing allogeneic mesenchymal stem cell therapy in androgenetic alopecia, with endpoints including hair density and patient-reported satisfaction. Success in these studies may expand treatment paradigms to include biologic and cell-based modalities, offering alternatives for patients unresponsive to conventional drugs.
Conclusion
The global hair treatment drug market is undergoing significant transformation, driven by advancements in pharmaceutical innovation, increasing prevalence of hair loss, and rising aesthetic awareness. With North America leading in current adoption and Asia Pacific poised for rapid growth, the market reflects both maturity and expansion potential.
The emergence of novel therapies such as JAK inhibitors, stem cell treatments, and improved topical formulations underscores a shift toward personalized and regenerative approaches. As regulatory oversight strengthens and clinical research progresses, the availability of safe, effective, and diverse treatment options is expected to expand, supporting robust and sustained market growth globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



