Table of Contents
Overview
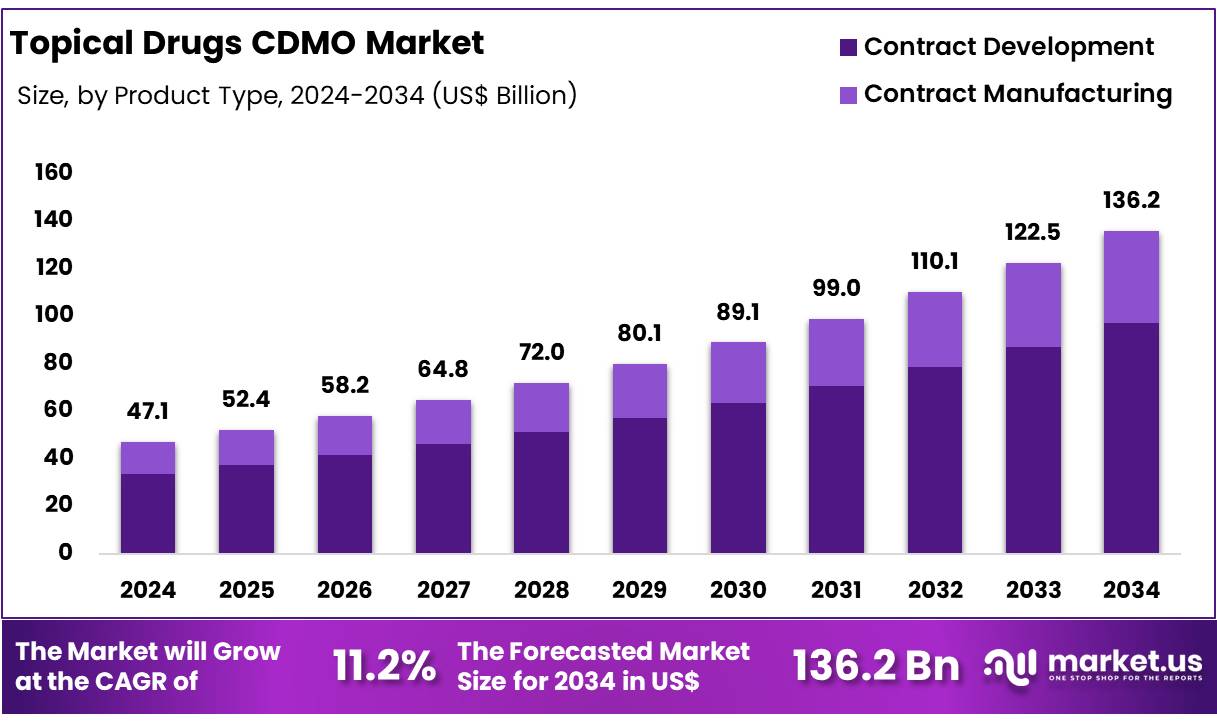
New York, NY – Nov 21, 2025 – Global Topical Drugs CDMO Market size is expected to be worth around US$ 136.2 Billion by 2034 from US$ 47.1 Billion in 2024, growing at a CAGR of 11.2% during the forecast period from 2025 to 2034. In 2024, Asia Pacific led the market, achieving over 37.2% share with a revenue of US$ 17.5 Billion.
The growth of the topical drugs contract development and manufacturing market has been supported by rising demand for advanced dermatology therapies and the increasing outsourcing preferences of pharmaceutical companies. Strong emphasis has been placed on efficient development workflows, high-quality production standards, and regulatory-compliant manufacturing systems to address expanding therapeutic needs in dermatology, pain management, and wound care.
A topical drugs CDMO is positioned to provide end-to-end solutions covering formulation development, analytical testing, scale-up, and commercial manufacturing. The service portfolio typically includes capabilities for creams, ointments, gels, lotions, foams, sprays, and transdermal systems. Investments in modern equipment and robust quality systems have strengthened the capacity to deliver consistent product performance across development and production stages.
The expansion of the global topical market has been attributed to increasing prevalence of skin disorders, aging populations, and growing adoption of self-administered therapies. As a result, pharmaceutical companies have shown strong interest in outsourcing to CDMOs with specialized expertise in semi-solid and liquid dosage forms. Strategic partnerships are being formed to accelerate time-to-market, reduce development risks, and ensure regulatory readiness.
The CDMO sector continues to demonstrate steady progress, driven by technological advancements, improved process efficiencies, and a rising focus on patient-centric formulations. Enhanced manufacturing flexibility and comprehensive support across the product lifecycle are expected to strengthen the role of topical drugs CDMOs in global pharmaceutical supply chains.
Key Takeaways
- The global market for topical drugs CDMO services is projected to reach US$ 136.2 billion by 2034, rising from US$ 47.1 billion in 2024.
- The market is anticipated to expand at a CAGR of 11.2% between 2025 and 2034.
- The semi-solid formulations segment is expected to represent 36.2% of the total market share in 2024.
- The contract manufacturing segment is projected to account for 69.2% of the market share in 2024.
- The dermatology therapeutic area is estimated to hold 39.5% of the total market share.
- The pharmaceutical companies end-use segment is expected to dominate with 39.5% of the market share.
- Asia Pacific is anticipated to lead the market in 2024, capturing over 37.2% of the total share.
Segmentation Analysis
- Product Type Analysis: Semi-solid formulations are expected to hold a dominant position in 2024, capturing 36.2% of the topical drugs CDMO market. The growth of this segment can be attributed to their effective delivery profiles through creams, gels, and ointments, which are widely utilized in dermatology and localized pain management. These formats support superior absorption and improved patient adherence. Although pastes, foams, and transdermal systems contribute to market expansion, their shares remain comparatively smaller. Solid and liquid formulations continue to represent limited portions of the market due to their reduced applicability in topical drug delivery.
- Service Analysis: The contract manufacturing segment is projected to lead the market in 2024 with a 69.2% share. This position is supported by the growing reliance of pharmaceutical firms on external manufacturing partners to reduce operational costs and access advanced production capabilities. Scalability, regulatory support, and accelerated commercialization timelines further reinforce the demand for contract manufacturing. While contract development services remain important for R&D activities and regulatory documentation, their overall market share is smaller due to their indirect contribution to commercial output.
- Therapeutic Area Analysis: Dermatology is anticipated to remain the largest therapeutic area in the topical drugs CDMO industry, representing 39.5% of the market in 2024. The high global incidence of dermatological disorders such as acne, eczema, and psoriasis continues to support the need for targeted topical therapies. Pain management and wound care segments also demonstrate steady growth but occupy smaller shares. Cosmetic dermatology and ophthalmology persist as niche categories with limited but emerging applications. Dermatology’s continued dominance is driven by sustained innovation and unmet needs in skin-focused therapeutics.
- End-use Analysis: Pharmaceutical companies are expected to account for 39.5% of the topical drugs CDMO market in 2024, maintaining the largest end-use share. This leadership is driven by extensive commercial manufacturing requirements and strategic outsourcing aimed at cost control and operational efficiency. Although biopharmaceutical companies are expanding their presence, their involvement remains comparatively lower due to their primary focus on biologics. Other stakeholders, including CROs and healthcare providers, hold minor shares and do not match the scale or procurement influence of pharmaceutical manufacturers.
Regional Analysis
North America is projected to retain a leading position in the topical drugs CDMO market in 2024, accounting for 37.2% of the global share. The region’s dominance is supported by strong demand for specialized topical therapies, driven by the rising incidence of dermatological disorders such as acne, eczema, and psoriasis. A mature pharmaceutical ecosystem has further encouraged the use of CDMO services, as companies increasingly seek cost-efficient and scalable development and manufacturing solutions.
Growth in North America is also reinforced by continuous advancements in formulation technologies, which have enabled the development of more effective and patient-centric topical products. The presence of a robust healthcare infrastructure and well-defined regulatory systems contributes to a favorable environment for CDMO operations, enhancing product quality and compliance.
In addition, increasing consumer interest in aesthetic and skincare products, including anti-aging and skin-brightening formulations, has supported market expansion in the region. As these factors persist, North America is expected to maintain its leadership in the global topical drugs CDMO market throughout 2024.
Frequently Asked Questions on Topical Drugs CDMO
- What services are offered by topical CDMOs?
Topical CDMOs provide formulation development, analytical testing, stability studies, packaging support, and commercial-scale manufacturing. These services enable pharmaceutical companies to reduce development timelines while ensuring regulatory compliance and product quality throughout the drug development cycle. - Why do pharmaceutical companies outsource topical drug development?
Outsourcing is driven by cost efficiency, access to specialized formulation expertise, and reduced operational risks. CDMOs allow companies to accelerate time-to-market while maintaining compliance with stringent quality standards required for topical drug products across global markets. - What dosage forms are commonly developed by topical CDMOs?
Topical CDMOs typically handle semi-solid and liquid forms such as creams, gels, ointments, lotions, foams, and solutions. These dosage forms require specific formulation knowledge to ensure proper skin penetration, therapeutic efficacy, and patient safety. - What factors influence the selection of a topical CDMO?
Selection depends on technical expertise, regulatory track record, manufacturing capacity, past performance, and geographic presence. Pharmaceutical companies also evaluate cost structure, technology capabilities, and flexibility in managing both early-stage development and commercial-scale production. - What is driving growth in the topical drugs CDMO market?
Market growth is supported by rising demand for dermatology treatments, increased outsourcing by pharmaceutical firms, and expanding chronic skin disease incidence. Growth is further reinforced by innovation in topical formulations requiring advanced technical capabilities. - Which therapeutic areas contribute most to market expansion?
Dermatology, pain management, wound care, and anti-inflammatory therapies are major contributors. Increasing prevalence of eczema, psoriasis, and acne promotes higher demand for specialized topical products manufactured through outsourced development partnerships. - What regions dominate the topical CDMO market?
North America and Europe hold strong market shares due to established pharmaceutical industries and advanced manufacturing infrastructure. Growth in Asia-Pacific is driven by cost-efficient production, expanding CDMO networks, and increasing investments in dermatology pipelines. - What trends are influencing future market growth?
Growing demand for personalized dermatology solutions, expansion in contract manufacturing, and advancements in formulation science guide market trends. Increased outsourcing by small and mid-sized pharmaceutical companies also reinforces long-term growth prospects across global regions.
Conclusion
The topical drugs CDMO market is expected to experience sustained expansion, supported by rising demand for advanced dermatology therapies, greater outsourcing activities, and continuous innovation in semi-solid and liquid formulations. Strong emphasis on regulatory compliance, efficient development pathways, and high-quality manufacturing has strengthened the role of CDMOs across the pharmaceutical value chain.
Increasing prevalence of skin disorders, aging populations, and adoption of patient-centric therapies further reinforce market growth. With Asia Pacific and North America emerging as key contributors, the sector is positioned to deliver scalable, flexible, and technologically advanced solutions that support accelerated commercialization and long-term industry development.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



