Table of Contents
Overview
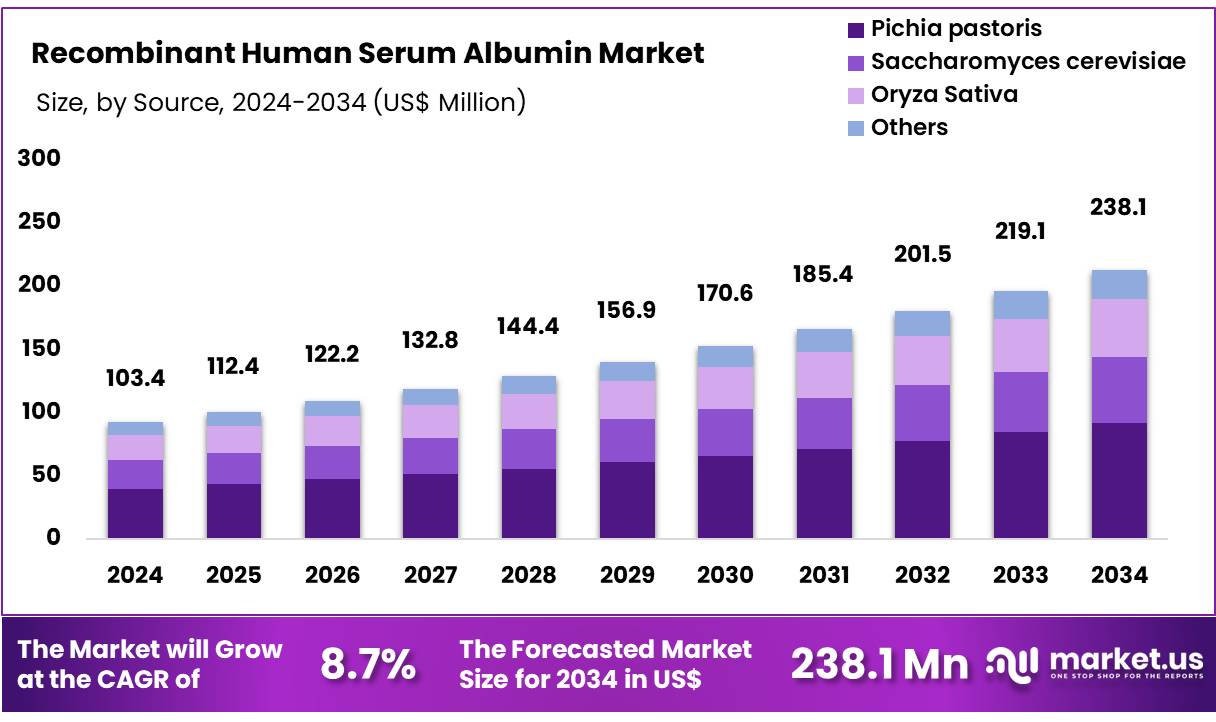
New York, NY – Nov 11, 2025 – Global Recombinant Human Serum Albumin Market was valued at US$ 103.4 Million in 2024 and is expected to grow at a CAGR of 8.7% from 2024 to 2034. In 2024, North America led the market, achieving over 35.6% share with a revenue of US$ 36.8 Million.
A new advancement in biopharmaceutical manufacturing has been marked by the introduction of high-quality Recombinant Human Serum Albumin (rHSA), developed to support the increasing demand for safe and consistent albumin sources in therapeutic, diagnostic, and cell-culture applications. The development of rHSA has been driven by the need for an alternative to plasma-derived albumin, as regulatory expectations for product safety and supply reliability have continued to rise across global healthcare markets.
The production of rHSA is based on recombinant technology, where targeted expression systems are utilized to generate albumin with high purity, structural stability, and functional equivalence to native human serum albumin. The process is designed to eliminate risks associated with blood-borne contaminants, and the controlled manufacturing environment ensures lot-to-lot consistency. This technological approach has strengthened the position of rHSA as a preferred ingredient in vaccine formulation, drug delivery systems, and cell preservation media.
Market adoption has been expanding, as industries have recognized the advantages of rHSA in improving formulation performance and enhancing overall product safety. Growth in regenerative medicine, biologics manufacturing, and advanced cell therapies is expected to further accelerate the use of recombinant albumin. Regulatory bodies have also demonstrated increasing acceptance of recombinant alternatives, reinforcing confidence in their clinical and commercial applications.
The launch of this rHSA product is expected to contribute to improved supply chain security and long-term sustainability within the biopharmaceutical sector, while supporting global efforts to advance innovation in life-science research and therapeutic development.
Key Takeaways
- The global recombinant human serum albumin market was valued at USD 103.4 million in 2024, and the market size is projected to reach USD 238.1 million by 2034. A compound annual growth rate of 8.7% is expected to be recorded during the forecast period.
- The pichia pastoris segment accounted for the largest share of the global market in 2024, representing 38.7% of total revenue.
- The cell culture media segment contributed the highest share among applications, capturing 34.5% of global revenue.
- The pharmaceutical and biotechnological industries segment dominated the end-use category, representing 54.5% of the total market share in 2024.
- North America remained the leading regional market, generating more than 35.6% of global revenue during the same year.
Regional Analysis
Demand for rHSA in North America has been driven by its extensive use in therapeutic applications, particularly in the management of chronic conditions such as diabetes and cancer. The product has been applied to improve the stability and performance of biologic drugs, including insulin formulations and cancer therapeutics, ensuring consistent efficacy throughout their shelf life.
Market expansion in the region has been supported by a recorded CAGR of 8.4%. Growth has been attributed to a strong biotechnological research landscape and a well-developed healthcare infrastructure. The U.S. and Canada have remained at the forefront of biopharmaceutical innovation, with a high concentration of advanced biotech companies and research institutions that continue to investigate and broaden the practical applications of rHSA.
Emerging Trends
- Alternative Production Methods: Production of human serum albumin has traditionally depended on donated blood, but recombinant approaches using plants and yeast are gaining importance. These platforms enable sustainable, large-scale manufacturing while reducing contamination risks associated with plasma-derived materials. Adoption of these alternative methods is becoming a major trend, supporting expanded biopharmaceutical applications.
- Use in Vaccines: Recombinant human serum albumin is increasingly applied as a stabilizer in vaccine formulations to maintain potency during storage and distribution. The Ebola vaccine ERVEBO, which incorporates rice-derived rHSA, illustrates this trend. Growing vaccine demand is expected to strengthen the use of rHSA, due to its role in improving product stability and overall effectiveness.
- Standardization Efforts: Standardization initiatives are progressing to ensure consistent quality in recombinant albumin products. Reference materials such as NIST’s SRM 2925 have been developed to support accuracy in testing and clinical use. These efforts enhance reliability across research and medical applications, reflecting rising emphasis on quality assurance within the biopharmaceutical sector.
Use Cases
- Medical Treatments: Recombinant human serum albumin is widely used to manage low albumin levels arising from burns, liver disorders, or surgical procedures. It supports blood volume and pressure stabilization, making it essential in critical care. Its therapeutic value is particularly evident in treating severe burn patients.
- Laboratory Research: In research settings, rHSA is applied to support cell culture systems by promoting cell viability and growth. Its use enhances efficiency in vaccine development and biologics production by optimizing culture conditions. These functions underline its significance in advancing biotechnological research.
- Medical Devices: Recombinant albumin is incorporated into medical devices to improve their functional performance. Applications include surgical sealants such as the Progel Pleural Air Leak Sealant, which enhances outcomes in lung procedures. This integration highlights the versatility of rHSA in supporting innovative healthcare solutions.
Frequently Asked Questions on Recombinant Human Serum Albumin
- What is Recombinant Human Serum Albumin (rHSA)?
Recombinant Human Serum Albumin is a laboratory-produced protein designed to replicate native human serum albumin. It is manufactured through recombinant technology to ensure high purity, improved safety, and reliable supply for pharmaceutical, clinical, and research applications. - How is rHSA produced?
rHSA is produced using engineered expression systems, most commonly pichia pastoris. These systems enable controlled synthesis of albumin with consistent structural and functional attributes, eliminating contamination risks associated with plasma-derived sources and improving overall manufacturing efficiency. - What are the key applications of rHSA?
The product is widely used in cell culture media, drug formulation, vaccine stabilization, and regenerative medicine. Its ability to enhance stability, solubility, and safety has supported its adoption in both clinical and industrial bioprocessing environments. - Why is recombinant albumin preferred over plasma-derived albumin?
Recombinant albumin is preferred because it eliminates pathogen transmission risks, provides consistent quality, and improves supply security. These advantages align with rising regulatory expectations for safety, purity, and traceability across biopharmaceutical production settings. - What factors are driving growth in the rHSA market?
Growth is driven by expanding biologics production, increasing adoption of cell-based therapies, and rising demand for safer drug-stabilizing excipients. Additional momentum is created by regulatory support for recombinant alternatives and advancements in bioprocessing technologies. - Which segment currently dominates the rHSA market?
The pharmaceutical and biotechnological industries segment holds the largest share due to extensive use of rHSA in drug formulation, biologics development, and advanced therapeutic manufacturing. This dominance is expected to persist as innovation in life sciences continues. - Which region leads the global rHSA market?
North America leads the market because of strong biopharmaceutical research capacity, well-established healthcare systems, and high adoption of advanced drug development technologies. Continued investment in biologics and cell therapy platforms supports sustained regional leadership.
Conclusion
The recombinant human serum albumin market is expected to advance steadily, supported by rising demand for safer and more reliable albumin sources across pharmaceutical, biotechnological, and clinical applications. Adoption is increasing as rHSA offers improved purity, consistency, and reduced contamination risks compared to plasma-derived alternatives.
Strong growth in biologics, vaccines, and cell-based therapies continues to reinforce its market position. North America remains a leading region due to its robust research ecosystem and established healthcare infrastructure. Expanding production technologies, standardization initiatives, and broader therapeutic and industrial use cases are expected to strengthen long-term market prospects.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



