Table of Contents
Overview
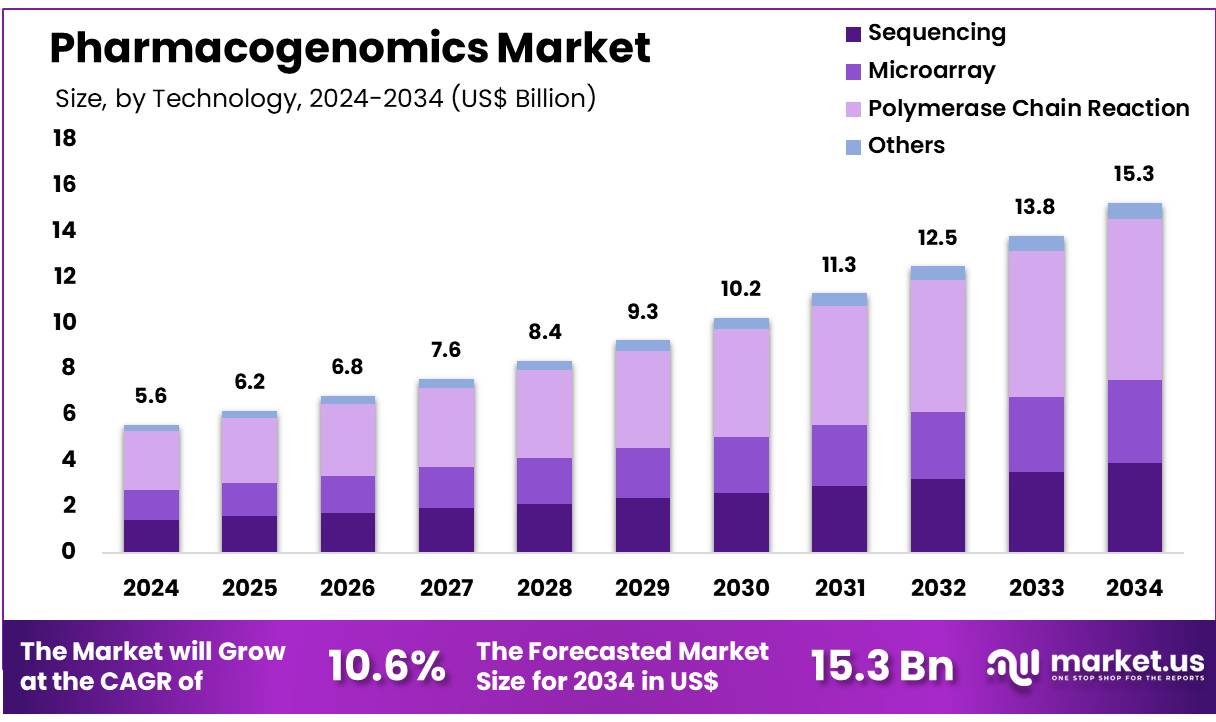
New York, NY – Nov 11, 2025 – The Global Pharmacogenomics Market was valued at US$ 5.58 Billion in 2024 and is expected to grow at a CAGR of 10.6% from 2024 to 2034. In 2024, North America led the market, achieving over 48.5% share with a revenue of US$ 2.7 Billion.
Pharmacogenomics has been recognized as a key driver in the transition toward more precise and individualized medical care. The field examines how genetic variation influences drug response, enabling treatments to be selected and adjusted according to a patient’s genetic profile. The adoption of pharmacogenomic testing has been supported by advances in genomic sequencing, bioinformatics, and clinical decision-support systems. As a result, medication management has been improved, and the risk of adverse drug reactions has been reduced across several therapeutic areas.
The global pharmacogenomics market has been experiencing steady expansion, as increasing demand for personalized therapies has been observed. Growth has also been supported by the rising prevalence of chronic diseases and the need for targeted drug optimization in oncology, cardiology, psychiatry, and infectious disease management. Regulatory agencies have encouraged the integration of genomic data into clinical practice, and several widely prescribed drugs now include pharmacogenomic guidance on their labels.
Significant investments have been directed toward research programs focused on the identification of drug-gene interactions. These initiatives have accelerated the development of companion diagnostics and strengthened the role of genetic testing in routine clinical workflows. Healthcare institutions have been expanding the use of pharmacogenomic panels to improve treatment accuracy and reduce healthcare costs associated with trial-and-error prescribing.
Ongoing innovation is expected to support continued market growth, as broader adoption in primary care and population health programs is anticipated. The increasing availability of cost-effective testing technologies and the establishment of large genetic databases have positioned pharmacogenomics as an essential component of future medical practice.
Key Takeaways
- The pharmacogenomics market generated revenue of US$ 5.6 Billion and is projected to reach US$ 15.3 Billion, supported by a CAGR of 10.6%.
- The polymerase chain reaction technology segment accounted for the largest revenue share, representing 45.9% of the market.
- The oncology application segment captured the highest revenue contribution, with a market share of 35.2%.
- The hospital and clinics end-user segment emerged as the leading contributor, accounting for 48.7% of total revenue.
- North America recorded the highest regional contribution, representing 48.5% of the total market share.
Pharmacogenomics Statistics
- Genetic influence on drug response has been estimated at up to 95%, indicating the substantial role of genomic variation in personalized medicine. This influence forms the basis for individualized treatment strategies aimed at improved therapeutic predictability.
- Approximately 20% of reported adverse drug reactions are associated with genetic factors. This association reinforces the importance of pharmacogenomics in reducing drug-related risks and enhancing medication safety across patient populations.
- Chronic pain management in the United States generates an annual economic burden ranging from USD 560 billion to USD 635 billion. The integration of pharmacogenomic approaches is expected to support cost reductions by improving therapeutic selection and treatment efficiency.
- Adverse drug reactions contribute to nearly 7% of hospital admissions and 20% of re-admissions and are ranked as the fourth leading cause of death in the United States. The associated financial burden is estimated at USD 136 billion annually.
- More than half of Medicare beneficiaries aged 65 years and older receive medications influenced by genetic variability. This trend highlights the growing relevance of pharmacogenomic testing in geriatric care and chronic disease management.
- Pharmacogenomics-guided modifications to medication regimens have been shown to reduce hospital admissions by up to 20%. These reductions support broader healthcare efficiency and contribute to improved patient outcomes.
- The U.S. Food and Drug Administration has approved more than 200 drug labels containing pharmacogenomic information. This approval base demonstrates the rising integration of genetic data into clinical decision-making frameworks.
- Large-scale initiatives, including the All of Us Research Program and the Pharmacogenomics Research Network, are accelerating the adoption of pharmacogenomics in healthcare. These initiatives promote the development of data resources and clinical implementation models.
- Ethical considerations remain central to the advancement of pharmacogenomics. Issues related to privacy, informed consent, and the potential for genetic discrimination must be addressed to support responsible deployment.
- Cost-effectiveness analyses indicate that pharmacogenomics contributes to reduced healthcare expenditures by optimizing medication selection and lowering the incidence of adverse drug reactions. These savings reinforce the long-term value of genetic-guided therapy.
- Ongoing education for healthcare professionals and the public is required to strengthen understanding of pharmacogenomics. Improved awareness is expected to support broader clinical adoption and informed patient participation.
- Continuous research and development efforts are necessary to expand the catalog of gene–drug interactions. Such advancements will support the evolution of personalized medicine and the identification of new therapeutic opportunities.
- Future potential remains significant, as pharmacogenomics is anticipated to transform clinical practice by enabling treatment strategies aligned with individual genetic profiles. This transformation is expected to enhance outcomes and reduce therapeutic variability.
Pharmacogenomics Application Analysis
- Drug Discovery: Pharmacogenomics supports drug discovery by identifying genetic determinants that influence drug metabolism, efficacy, and safety. These insights facilitate the development of targeted therapies that align with specific genetic profiles. Reference resources, such as the FDA’s biomarker table, illustrate the growing reliance on genomic information in drug development.
- Infectious Diseases: Within infectious disease management, pharmacogenomics contributes to the understanding of how genetic variants influence responses to antimicrobial agents. This understanding enables the optimization of dosing strategies and therapeutic selection. Support from programs led by NIAID strengthens research efforts aimed at improving treatment outcomes for infectious pathogens.
- Oncology: Oncology represents one of the most advanced areas of pharmacogenomic application. Genetic profiling of both patients and tumors informs the development of individualized cancer therapies, resulting in improved efficacy and reduced toxicity. Global initiatives supported by the NIH emphasize the translation of genomic research into clinical oncology practice.
- Cardiovascular Diseases: In cardiovascular care, pharmacogenomics informs the selection and dosing of medications such as anticoagulants and statins. Genetic insights support improved safety and therapeutic precision. FDA guidance on combination drug development reflects the increasing importance of genomic data in cardiovascular treatment planning.
- Other Applications: Broader applications of pharmacogenomics extend to psychiatry, neurology, and pediatrics. Genetic information is used to customize therapies for mental health conditions, neurological disorders, and age-specific needs in pediatric care. FDA research initiatives continue to highlight the importance of incorporating genomic considerations into drug development for diverse patient populations.
Regional Analysis
The North American region is recognized as the leading market for pharmacogenomics, accounting for 48.5% of global revenue, and is anticipated to retain its dominant position over the forecast period. The expansion of the market is supported by the increasing incidence of chronic conditions such as cancer, cardiovascular disorders, and neurological diseases.
Pharmacogenomics, which involves the use of genetic testing to identify specific mutations, has supported clinical decision-making by enabling oncologists to select appropriate targeted therapies and assess patient-specific treatment responses. This advancement has contributed to the rising adoption of pharmacogenomic testing across healthcare facilities.
According to the International Agency for Research on Cancer, North America reported a cancer incidence rate of 364.7 per 100,000 individuals in 2022, which has increased the demand for pharmacogenomic testing in countries such as the United States and Canada.
Continuous innovation in genomic technologies is also enhancing regional market growth. The emergence of cost-effective, scalable, and portable nucleic acid sequencing platforms, including those developed by PacBio and Oxford Nanopore, is expected to significantly influence the market over the next five years.
Growing utilization of pharmacogenomics in drug discovery and clinical research is further contributing to market expansion. In addition, regional initiatives aimed at promoting pharmacogenomic testing, such as the Canadian Pharmacogenomics Network for Drug Safety, are supporting efforts to minimize adverse drug reactions in both pediatric and adult populations.
Frequently Asked Questions on Pharmacogenomics
- How does pharmacogenomics improve treatment outcomes?
Improved treatment outcomes are achieved through the identification of genetic markers that affect drug metabolism and response. Personalized dosing strategies are supported, which helps reduce therapeutic failures and adverse reactions while enhancing safety and clinical effectiveness in routine medical practice. - Why is pharmacogenomics important in clinical decision-making?
Pharmacogenomics is considered important because it supports evidence-based decisions for drug selection and dosing. Genetic insights guide clinicians toward individualized therapy pathways, which contributes to more predictable responses and optimized patient management across diverse healthcare settings. - What diseases benefit most from pharmacogenomic testing?
Pharmacogenomic testing is widely applied in oncology, cardiology, psychiatry, and infectious diseases. Treatment regimens in these fields are influenced strongly by genetic variations, which allows physicians to adjust drugs and dosages for improved safety and therapeutic effectiveness. - How is pharmacogenomic information obtained?
Genetic information is derived from laboratory testing using blood or saliva samples. These tests identify gene variants involved in drug metabolism, transport, or receptor activity. The generated insights guide personalized therapeutic strategies in alignment with evidence-based clinical recommendations. - Are pharmacogenomic tests widely available?
Availability has increased due to technological advancements and reduced sequencing costs. Many healthcare systems provide targeted gene panels, while some institutions integrate pharmacogenomic testing into routine practice for high-risk drugs associated with substantial variability in patient response. - What factors are driving growth in the pharmacogenomics market?
Market growth is attributed to rising demand for precision therapeutics, declining sequencing costs, and increasing awareness of adverse drug reactions. Expanded integration of genomic data into clinical workflows also supports stronger revenue growth in testing and analytical solutions. - Which regions dominate the pharmacogenomics market?
North America maintains a dominant position because of advanced healthcare systems, strong research investments, and early adoption of genetic testing. Europe and Asia Pacific demonstrate accelerated growth as personalized medicine initiatives expand across public and private healthcare sectors.
Conclusion
A sustained shift toward personalized medicine has been reinforced by the expanding use of pharmacogenomic testing, and steady market growth has been supported by technological progress, rising chronic disease prevalence, and broader clinical integration. Increased adoption in oncology, cardiology, psychiatry, and infectious diseases is expected as health systems prioritize treatment precision and safety.
Regulatory support, large-scale genomic initiatives, and declining sequencing costs are strengthening commercialization and clinical uptake. The market outlook remains positive, as pharmacogenomics is anticipated to become a core component of routine care, improving therapeutic outcomes while reducing the economic burden associated with adverse drug reactions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



