Table of Contents
Overview
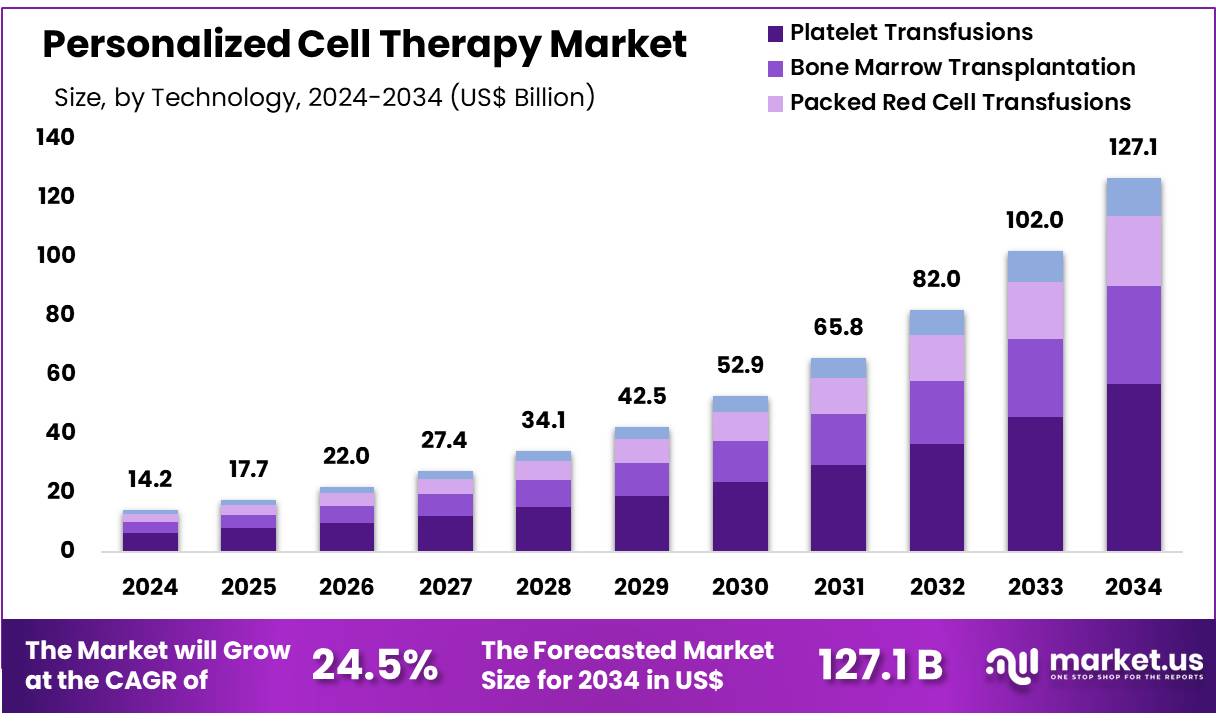
New York, NY – Oct 31, 2025 – Global Personalized Cell Therapy Market size is expected to be worth around US$ 127.1 Billion by 2034 from US$ 14.2 Billion in 2024, growing at a CAGR of 24.5% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 41.2% share with a revenue of US$ 5.9 Billion.
A significant advancement in personalized medicine has been introduced with the development of next-generation personalized cell therapy, designed to transform the treatment paradigm for chronic and life-threatening diseases. The platform has been engineered to utilize patient-specific cells, enabling targeted therapeutic responses that minimize adverse effects and improve long-term clinical outcomes.
Personalized cell therapy has emerged as a critical innovation in biotechnology, supported by strong clinical evidence that demonstrates enhanced safety profiles and improved therapeutic efficacy. This growth can be attributed to increasing investments in regenerative medicine, rising incidences of cancer and genetic disorders, and advancements in cellular engineering technologies. Strategic collaborations among biotechnology firms, research institutions, and healthcare providers have further accelerated product development and commercialization.
The newly launched therapy platform integrates advanced cell-processing techniques, automated manufacturing systems, and rigorous quality-control protocols. As a result, treatment timelines are reduced, and cell viability and reproducibility are significantly enhanced. The approach enables scalable production models, positioning the therapy for broader adoption across global healthcare systems.
The company remains committed to expanding its clinical pipeline, collaborating with regulatory agencies, and strengthening global partnerships to ensure timely patient access. Continued research and development initiatives will support future indications across oncology, immunology, and rare disease treatment.
This milestone reflects a growing commitment to innovation in personalized therapeutics and reinforces the vision of delivering life-changing treatment solutions tailored to individual patient needs.
Key Takeaways
- In 2024, the Personalized Cell Therapy market recorded revenue of US$ 14.2 billion. The market is projected to expand at a CAGR of 24.5%, reaching US$ 127.1 billion by 2034.
- The therapeutic area segment comprises neurological disorders, cardiovascular diseases, inflammatory diseases, diabetes, and cancer. Cardiovascular diseases accounted for the largest share in 2024, representing 48.7% of the market.
- Based on technology, the market includes bone marrow transplantation, platelet transfusions, packed red cell transfusions, and organ transplantation. Platelet transfusions dominated the segment with a 44.8% share.
- By end user, the market is categorized into hospitals, specialty clinics, homecare, and others. Hospitals held the leading position, contributing 58.2% of total market revenue.
- North America emerged as the leading regional market in 2024, capturing 41.2% of the overall share.
Regional Analysis
North America remains the leading region in the Personalized Cell Therapy market
North America accounted for the largest revenue share of 41.2%, driven by rapid advancements in immunotherapy, increased investments in cell therapy research, and growing demand for targeted therapeutic approaches. Personalized cell therapies, including CAR T-cell therapies and stem cell-based treatments, have shown significant success in managing cancers and autoimmune disorders, reinforcing regional market dominance.
A notable development took place in August 2024, when Adaptimmune Therapeutics PLC received U.S. FDA approval for TECELRA (afamitresgene autoleucel), marking the first engineered cell therapy authorized for the treatment of solid tumors and synovial sarcoma, a rare soft-tissue malignancy. This regulatory milestone reflects the expanding therapeutic potential of personalized cell approaches and their ability to target cancer cells more precisely than traditional treatment modalities.
The region’s leadership is further supported by the high prevalence of cancer and chronic illnesses, advancements in genetic engineering, and the widespread adoption of personalized medicine tailored to individual genetic profiles.
Asia Pacific is poised to register the highest growth rate during the forecast period
The Asia Pacific market is expected to grow at the fastest CAGR, supported by advancements in biotechnology, rising healthcare expenditure, and increasing emphasis on precision medicine. Countries such as China, India, and Japan are projected to experience heightened demand for cell-based therapies as healthcare systems evolve and access to advanced treatments expands. The growing burden of cancer, autoimmune conditions, and genetic disorders is anticipated to accelerate adoption across the region.
China’s significant investments in biotechnology and initiatives in stem cell and gene therapy research are expected to contribute prominently to market development. Enhanced healthcare infrastructure, increasing collaborations between international pharmaceutical organizations and regional biotech firms, and growing awareness of individualized treatment benefits are expected to propel innovation and adoption.
As precision-based approaches gain greater traction, the Asia Pacific Personalized Cell Therapy market is projected to achieve substantial expansion over the coming years.
Emerging Trends
- Evolving Regulatory Frameworks: In January 2024, the U.S. FDA issued updated guidance documents for human gene-editing and CAR-T therapies, aiming to clarify development standards. These updates addressed genome-editing protocols and safety evaluations for allogeneic cell products, indicating a regulatory effort to align oversight with rapid scientific advancements.
- Accelerated Therapy Approvals: As of May 15, 2025, the FDA had approved 45 cell and gene therapies, including CAR-T treatments, engineered tissues, and gene-replacement products. This represents a substantial increase from a limited number of authorized therapies five years earlier, illustrating rapid commercialization and clinical progress.
- Major NIH Funding Support: In October 2024, the NIH allocated USD 14 million through its Somatic Cell Genome Editing Program to support prime-editing technology development. The initiative targets creation of personalized therapies for urea cycle disorders, which affect approximately one in 35,000 children, highlighting emphasis on rare disease innovation.
- Expanding Development Pipeline: The American Society for Gene & Cell Therapy reported 4,238 gene, cell, and RNA-based therapies in active development across the United States in 2024. This pipeline volume reflects strong research momentum and diversification of therapeutic modalities under clinical and preclinical evaluation.
- Notable Solid-Tumor Progress: A National Cancer Institute study conducted in July 2024 showed promising results for personalized cellular immunotherapy in metastatic colorectal cancer, with three out of seven patients achieving tumor reduction and a median progression-free survival of 4.6 months. These data represent early but meaningful progress in solid-tumor applications.
Use Cases
- CAR-T Therapies for Hematologic Malignancies: Seven CAR-T products have been approved by the FDA for treatment of leukemias, lymphomas, and multiple myeloma. These autologous T-cell therapies target markers such as CD19 and BCMA, delivering remission rates approaching 80 percent in patients with treatment-resistant disease.
- Tumor-Infiltrating Lymphocyte (TIL) Therapy: In an NCI-led clinical study, metastatic colorectal cancer patients received expanded tumor-infiltrating lymphocytes. Three of seven participants demonstrated meaningful tumor reduction lasting up to seven months, with a median time to progression of 4.6 months, indicating emerging promise in solid-tumor immunotherapy.
- Prime Editing for Rare Metabolic Disorders: A USD 14 million NIH grant is supporting CRISPR-based prime-editing strategies to treat ultra-rare urea cycle disorders. These personalized genetic correction therapies are being developed for a population with an estimated incidence of one in 35,000 children, underscoring high-impact pediatric innovation.
- iPSC-Based Treatments for Parkinson’s Disease: NIH funding totaling USD 2.06 million is enabling three research programs advancing induced pluripotent stem cell approaches for Parkinson’s disease. The work focuses on replacing lost neurons with patient-derived cells, representing a personalized strategy to address neurodegeneration.
Frequently Asked Questions on Personalized Cell Therapy
- How does personalized cell therapy work?
The therapy involves isolating specific cells from the patient, expanding or engineering them ex vivo, and administering them back into the body. The approach enables precise biological repair and reduces immune rejection risks compared with conventional therapeutics. - What diseases are treated with personalized cell therapy?
Applications include cancer, autoimmune disorders, cardiovascular disease, and degenerative neurological conditions. Prominence has been observed in oncology, particularly hematological malignancies, where adoptive cell therapies such as CAR-T have demonstrated significant clinical efficacy. - What are the major benefits of personalized cell therapy?
Benefits include targeted disease management, reduced immune rejection, long-term therapeutic effect potential, and minimal toxicity. The therapy enhances precision medicine adoption and strengthens patient-specific biological repair mechanisms. Clinical outcomes have shown encouraging therapeutic durability. - What is driving growth in the personalized cell therapy market?
Market expansion is driven by rising demand for precision medicine, increased prevalence of chronic diseases, technological advancements in cell engineering, and favorable clinical results. Government support and investment by biotechnology firms further accelerate commercialization momentum. - Which regions dominate the personalized cell therapy market?
North America and Europe currently lead due to advanced healthcare infrastructure, supportive regulatory frameworks, and significant R&D investments. Asia-Pacific is emerging rapidly, supported by government funding, rising clinical trials, and expanding biopharmaceutical manufacturing capabilities. - What future trends are expected in the market?
Future growth will be supported by automation in cell processing, AI-assisted therapy design, decentralized manufacturing models, and improved regulatory pathways. Strategic collaborations between biotech firms, hospitals, and academic institutes are expected to enhance innovation and scalability.
Conclusion
The personalized cell therapy landscape is experiencing rapid scientific and commercial advancement, driven by strong clinical progress, increasing regulatory clarity, and expanding investment in advanced cellular engineering.
Market growth is supported by rising prevalence of chronic and genetic diseases, robust research pipelines, and growing adoption of precision medicine approaches across major healthcare markets. Regional leadership from North America and accelerating momentum in Asia Pacific reinforce global expansion potential.
Continued regulatory support, technological upgrades in automated manufacturing, and increasing collaboration between industry and research institutions are expected to strengthen scalability and patient access. Personalized cell therapy is positioned to transform long-term disease management worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



