Table of Contents
Overview
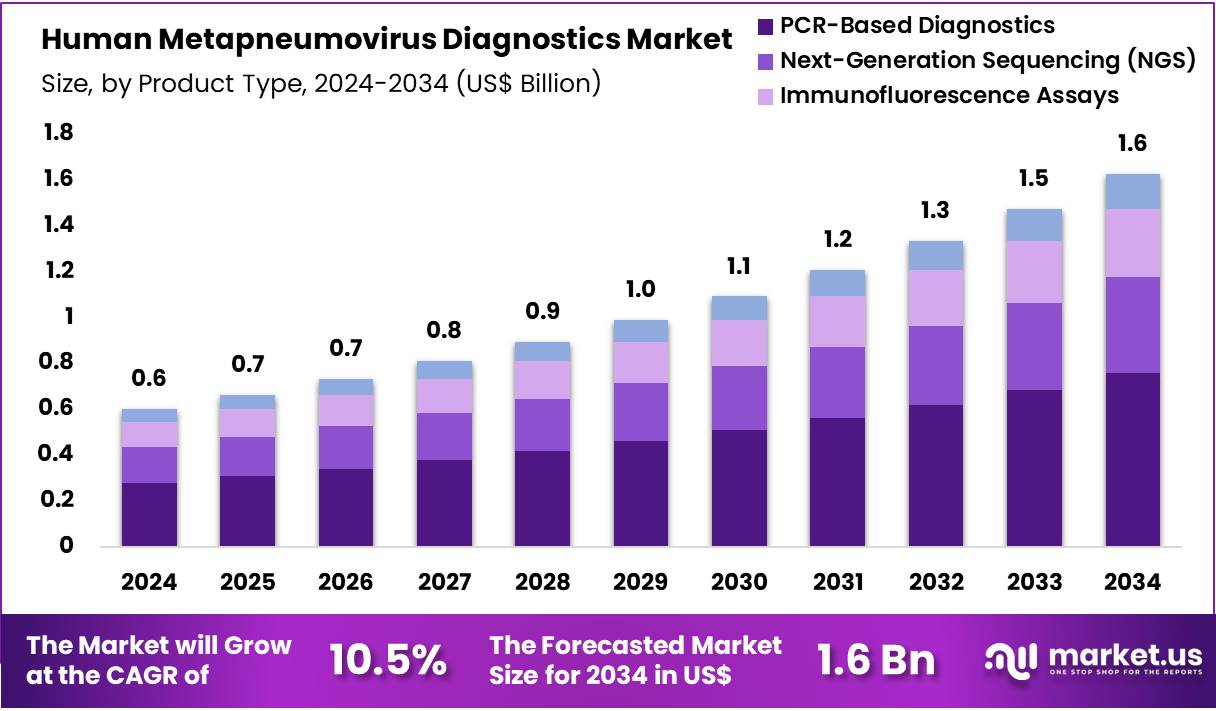
New York, NY – Nov 07, 2025 – The Global Human Metapneumovirus Diagnostics Market size is expected to be worth around US$ 1.6 Billion by 2034 from US$ 0.6 Billion in 2024, growing at a CAGR of 10.5% during the forecast period 2025 to 2034.
The global demand for Human Metapneumovirus (HMPV) diagnostics has been increasing as respiratory infection surveillance is strengthened across healthcare systems. HMPV is recognized as a significant cause of acute respiratory illness, particularly among infants, older adults, and immunocompromised individuals. The growing awareness of its clinical impact has resulted in heightened adoption of advanced diagnostic technologies.
The market has been shaped by the rising use of molecular diagnostic methods. Polymerase Chain Reaction (PCR) assays have been widely utilized due to their high sensitivity and rapid turnaround time. The growth of the market has been supported by the integration of multiplex PCR platforms that enable simultaneous detection of multiple respiratory pathogens, improving diagnostic accuracy and workflow efficiency in clinical laboratories.
The expansion of point-of-care testing is contributing further to improved disease management. Rapid antigen detection tests are increasingly utilized in emergency care settings, allowing timely clinical decisions and reducing the burden on centralized laboratories. The adoption of automated systems by hospitals and diagnostic centers has also strengthened testing throughput during peak respiratory seasons.
The market growth is driven by expanding research activities, increased healthcare spending, and the rise in respiratory infection surveillance programs. The importance of early and accurate diagnosis has been emphasized following recent global health events, and HMPV testing has been incorporated into broader respiratory virus panels to support comprehensive patient evaluation.
Overall, the Human Metapneumovirus diagnostics landscape is expected to experience steady expansion, supported by technological advancements, improved laboratory infrastructure, and the growing prioritization of respiratory disease detection worldwide.
Key Takeaways
- In 2024, the Human Metapneumovirus diagnostics market generated revenue of US$ 6 Billion. The market has been expanding at a CAGR of 10.5% and is projected to reach US$ 1.6 Billion by 2033.
- The product landscape has been categorized into PCR-based diagnostics, next-generation sequencing (NGS), immunofluorescence assays, and other emerging modalities. PCR-based diagnostics accounted for the largest contribution in 2024, representing 46.5% of total revenue.
- Based on end use, the market is segmented into hospitals and clinics, diagnostic and reference laboratories, academic and research institutions, and other healthcare settings. Hospitals and clinics were the dominant users, capturing 52.8% of overall demand in 2024.
- From a regional perspective, North America*remained the leading market, supported by well-established healthcare infrastructure and advanced diagnostic adoption, and accounted for 39.5% of global revenue in 2024.
Regional Analysis
North America is leading the Human Metapneumovirus Diagnostics Market
North America held the largest share of the Human Metapneumovirus (hMPV) diagnostics market, accounting for 39.5% of total revenue. The growth in the region has been attributed to rising awareness of respiratory infections and increasing demand for rapid diagnostic solutions. The acquisition of Icosavax, Inc. by AstraZeneca in December 2023 strengthened the region’s focus on vaccine and diagnostic innovation, particularly through the integration of IVX-A12, a potential first-in-class combination vaccine targeting RSV and hMPV.
The increasing incidence of respiratory infections among older adults and immunocompromised populations has elevated the need for early and accurate diagnostic tools. Advancements in molecular diagnostics, including PCR-based platforms and point-of-care testing, have improved detection capabilities and supported overall market expansion. Investments in healthcare infrastructure and ongoing research initiatives have further encouraged the development of advanced diagnostic assays.
Partnerships between biotechnology companies and healthcare providers have broadened access to high-quality testing solutions. Additionally, public health programs promoting early detection and awareness have contributed to the widespread use of hMPV diagnostic tests across the U.S. and Canada.
Asia Pacific is expected to exhibit the fastest growth
The Asia Pacific region is projected to record the highest CAGR during the forecast period, driven by rising healthcare expenditure and increasing cases of respiratory infections. Expanding healthcare infrastructure in China, India, and Japan is expected to enhance access to advanced diagnostic technologies. Government initiatives supporting infectious disease monitoring and early detection are anticipated to stimulate adoption rates.
Increasing demand for point-of-care and molecular testing across both urban and rural settings is expected to support market growth. Collaborations between global diagnostic manufacturers and regional research institutions are improving the availability and affordability of innovative assays. Growing public awareness of respiratory illnesses and their impact on vulnerable populations is also contributing to rising diagnostic demand.
Advancements in multiplex testing, enabling simultaneous detection of multiple respiratory viruses, are expected to improve diagnostic accuracy and efficiency. The integration of artificial intelligence into laboratory workflows is further enhancing the precision and speed of hMPV detection, reinforcing market growth across Asia Pacific.
Emerging Trends
- Increased Adoption of Molecular Testing: The use of molecular diagnostic technologies, particularly reverse transcription polymerase chain reaction (RT-PCR), has expanded considerably. These methods are characterized by high sensitivity and specificity, enabling the reliable detection of hMPV even at low viral concentrations. The deployment of multiplex RT-PCR assays has supported simultaneous identification of hMPV and other respiratory pathogens, improving the efficiency of respiratory illness diagnostics.
- Advancements in Rapid Antigen Detection: Rapid antigen detection methods for hMPV are gaining attention due to the need for accelerated clinical decision-making. Although these tests currently demonstrate lower sensitivity compared to molecular assays, ongoing development efforts are focused on enhancing their performance. The aim is to deliver faster and more accurate point-of-care testing solutions.
- Integration of Next-Generation Sequencing: Next-generation sequencing is being evaluated for its ability to characterize hMPV strains in detail. This technology provides comprehensive genomic insights that support the study of viral evolution, transmission pathways, and potential therapeutic targets. While not yet integrated into routine diagnostics, its long-term applicability for surveillance and outbreak investigation is considered promising.
- Increased Focus on Seasonal Surveillance: Surveillance activities show that hMPV infections typically rise in late winter and spring. Understanding these seasonal trends supports more accurate differential diagnosis, particularly when respiratory symptoms are present but tests for common pathogens such as influenza and RSV yield negative results.
Use Cases
- Hospitalized Patients with Acute Respiratory Infections: Accurate hMPV detection remains essential for hospitalized patients presenting with acute respiratory infections. Evidence indicates that approximately 6.24 percent of such cases are linked to hMPV, underscoring the need for its inclusion in diagnostic protocols to optimize treatment strategies and infection control.
- Pediatric Populations: Children under five years of age represent a high-risk group for hMPV infection. The virus is associated with significant lower respiratory tract conditions, including bronchiolitis and pneumonia. Early diagnostic confirmation supports timely clinical management and reduces transmission in community environments such as daycare centers and schools.
- Elderly and Immunocompromised Individuals: Older adults and immunocompromised patients exhibit elevated vulnerability to severe hMPV-related complications. Early identification through sensitive molecular methods assists in improving clinical outcomes and may help limit hospitalization requirements.
- Outbreak Monitoring in Long-Term Care Facilities: Long-term care facilities are highly susceptible to respiratory virus outbreaks. Incorporating routine hMPV testing during such events enables rapid identification of the causative pathogen and supports targeted containment strategies to protect residents and staff.
- Public Health Surveillance: The integration of hMPV diagnostics into national respiratory virus monitoring systems strengthens epidemiological understanding. The resulting data contribute to informed public health planning, the development of preventive measures, and more efficient allocation of resources during peak respiratory illness periods.
Frequently Asked Questions on Human Metapneumovirus Diagnostics
- How is hMPV detected in clinical settings?
Detection is performed through molecular assays such as PCR, antigen tests, and immunofluorescence methods. PCR-based diagnostics are preferred due to their high sensitivity and ability to identify viral genetic material accurately, even in early infection stages. - Why is early diagnosis of hMPV important?
Early diagnosis supports timely patient management, reduces unnecessary antibiotic use, and helps clinicians differentiate hMPV from other respiratory viruses. Accurate detection also enhances infection control measures and supports targeted public health interventions during peak respiratory seasons. - Which diagnostic method is considered most accurate?
PCR testing is regarded as the most accurate method because it directly identifies viral RNA with high sensitivity. Its ability to detect low viral loads makes it essential for reliable clinical confirmation of hMPV infection across diverse patient groups. - Who should be tested for hMPV?
Testing is recommended for patients with severe respiratory symptoms, high-risk individuals, and those with unexplained lower respiratory tract infections. Healthcare facilities may also test during outbreaks to support surveillance and prevent the spread of respiratory illness. - Are point-of-care tests available for hMPV?
Yes, rapid point-of-care tests are increasingly available to support immediate clinical decision-making. These tests are useful in emergency and outpatient settings, delivering quicker results while reducing the burden on centralized diagnostic laboratories. - Which diagnostic segment holds the largest market share?
PCR-based diagnostics dominate the market due to their accuracy, efficiency, and widespread adoption in hospitals and laboratories. Their ability to detect multiple respiratory pathogens simultaneously further reinforces their leading position within the global diagnostic landscape. - Which end-use segment contributes most to market revenue?
Hospitals and clinics account for the largest market share because they perform the highest volume of respiratory testing. Their access to advanced diagnostic equipment and need for rapid patient management drive consistent adoption of hMPV diagnostic solutions. - Which region leads the global hMPV diagnostics market?
North America leads the market due to strong healthcare infrastructure, high diagnostic adoption, and increased public awareness. Investments in molecular diagnostics and strategic collaborations between biotechnology firms further strengthen the region’s dominant position.
Conclusion
The global Human Metapneumovirus diagnostics market is expected to maintain steady growth, supported by rising respiratory infection surveillance, expanding molecular testing adoption, and increasing healthcare investments. The dominance of PCR-based diagnostics, the emergence of rapid antigen and NGS technologies, and the integration of multiplex platforms continue to enhance diagnostic accuracy and efficiency.
North America leads the market due to advanced infrastructure, while Asia Pacific is projected to experience the fastest expansion. Growing awareness of hMPV’s clinical impact, coupled with strengthened laboratory capabilities and broader access to point-of-care solutions, is expected to support sustained market development over the forecast period.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



