Table of Contents
Overview
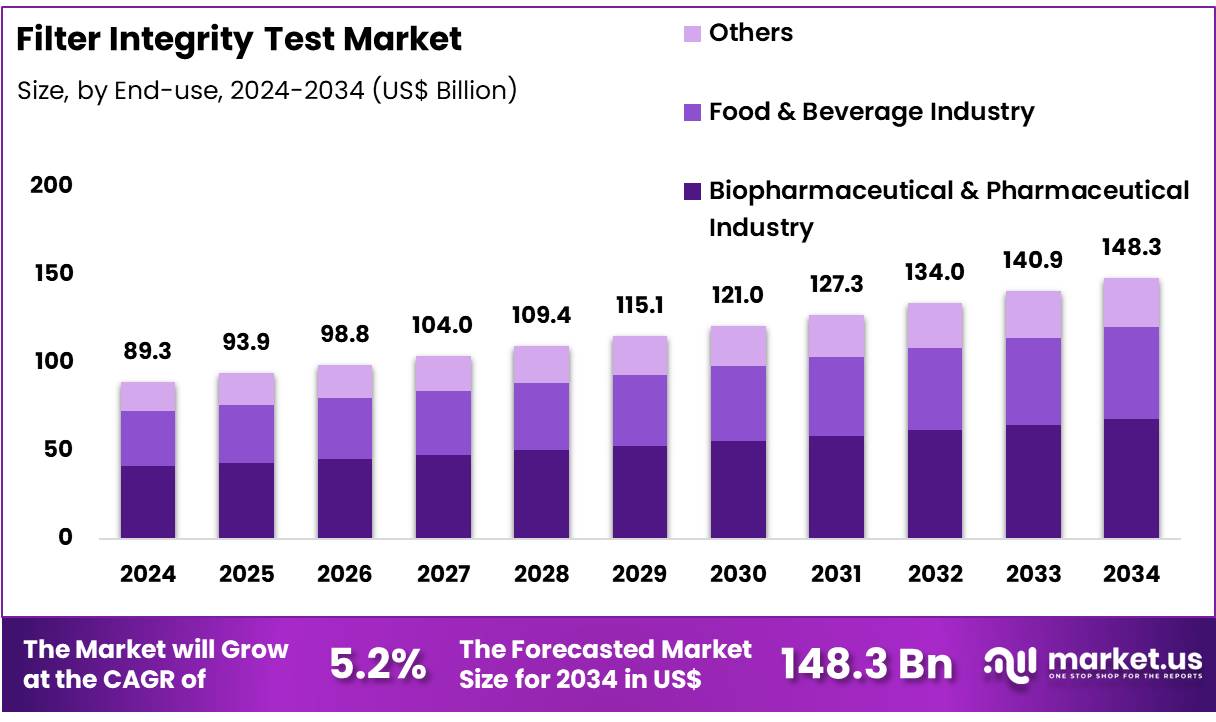
New York, NY – Nov 28, 2025 – Global Filter Integrity Test Market size is expected to be worth around US$ 148.3 Billion by 2034 from US$ 89.3 Billion in 2024, growing at a CAGR of 5.2% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 41.8% share with a revenue of US$ 37.3 Billion.
A new Filter Integrity Test protocol has been introduced to support improved reliability and operational assurance across critical filtration applications. The formation of this protocol has been designed to establish a standardized method for assessing filter performance, structural stability, and contamination-control efficiency under defined operating conditions. The procedure has been structured to evaluate key parameters such as pressure resistance, flow behaviour, and particulate retention, ensuring that each unit complies with required quality benchmarks.
The test method has been developed to verify that filter systems maintain their integrity throughout their service life. The process includes pre-test inspection, controlled challenge introduction, differential pressure measurement, and post-test verification. Through this structured approach, manufacturers and end-users are expected to achieve improved traceability, enhanced validation accuracy, and reduced operational risks.
The introduction of this integrity test is expected to support industries where filtration reliability is essential, including pharmaceuticals, biotechnology, food processing, microelectronics, and industrial water treatment. The increased focus on product safety and regulatory compliance has created a strong need for repeatable and verifiable filter-testing methods. The newly established protocol has been positioned to address these requirements by providing a consistent and transparent framework.
This basic formation is intended to guide laboratories, production facilities, and quality-assurance teams in implementing standardized evaluation procedures. The approach has been aligned with global quality expectations and is anticipated to contribute to higher operational safety and improved product-quality outcomes.
Key Takeaways
- The global filter integrity test market reached a valuation of USD 89.3 billion in 2024 and is projected to grow to USD 148.3 billion by 2034, reflecting a CAGR of 5.2%.
- The bubble point test category dominated the market in 2024, accounting for 33.5% of total revenue.
- Liquid filter integrity testing represented the largest share of the market, contributing 56.8% of overall revenue.
- The biopharmaceutical and pharmaceutical industry segment held the leading position, capturing 60.5% of global revenue.
- North America remained the foremost regional market, with more than 41.8% of total revenue.
Segmentation Analysis
- Test Method Analysis: In 2024, the bubble point test segment accounted for 33.5% of global filter integrity testing revenue, maintaining its position as the leading method. This dominance can be attributed to its high reliability and precision in identifying micro-defects within filter membranes. The method is extensively applied in pharmaceutical, food, and water processing sectors to ensure that pore sizes comply with sterilization requirements. Its non-destructive nature, coupled with increasing demand for high-quality sterile products, has strengthened adoption. Advancements in automation and sensor technologies have further enhanced measurement accuracy, supporting product sterility and regulatory compliance.
- Type Analysis: The liquid filter integrity test segment held a 56.8% share of global revenue in 2024, positioning it as the dominant segment. This method plays a critical role in biopharmaceutical, food, and water applications by confirming sterility and particle retention capabilities of filtration systems. Techniques such as forward flow and bubble point testing are utilized to ensure compliance with stringent FDA and EMA standards. The integration of automated testing platforms has improved precision and minimized operator-related variability. As a result, liquid testing remains the preferred approach for ensuring product quality, regulatory alignment, and operational efficiency.
- End-use Analysis: In 2024, the biopharmaceutical and pharmaceutical industry segment led the market with a 60.5% share. This leadership is driven by rigorous regulatory requirements and the heightened need for sterility assurance in biologics, vaccines, and other high-value therapeutics. Commonly employed methods, including bubble point and forward flow tests, are essential for verifying filter performance and microbial retention. Increasing levels of automation have facilitated real-time monitoring and comprehensive data analytics. Growing emphasis on biologics manufacturing and low-bioburden production environments continues to reinforce the strategic importance of integrity testing in overall quality assurance frameworks.
Emerging Trends
The utilization of deterministic test methods has been increasing, as these approaches provide measurable, objective results and exhibit greater sensitivity when compared with probabilistic techniques.
Regulatory expectations outlined in USP <1207> and reinforced through recent FDA Annex 1 revisions have been promoting risk-based integrity testing across the entire product lifecycle.
The implementation of pre-use post-sterilization integrity testing (PUPSIT) has been expanding, ensuring that filter integrity is verified immediately before critical manufacturing steps.
The rise of single-use systems (SUS) has been accelerating the adoption of in-place integrity testing, with filters typically removed after processing a single lot or after one operational day.
Digital automation and inline monitoring solutions are emerging, enabling real-time data acquisition while reducing manual handling in aseptic production environments.
Use Cases
Sterile Drug Manufacturing: Sterilizing-grade filters used for drug solution processing are subjected to bubble-point or pressure-hold tests designed to identify defects at failure rates below 0.01%. HEPA filters in aseptic rooms are evaluated biannually to confirm particle retention efficiencies of at least 99.97% for particles ≥0.3 µm.
Container Closure Integrity (CCI): Instead of routine sterility tests, CCI assessments are performed annually and again at product expiry to confirm that primary packaging maintains the maximum allowable leakage limit (MALL) throughout the product’s shelf life.
Pre-Use Post-Sterilization Integrity Testing (PUPSIT): Prior to each production batch, filters and related assemblies undergo PUPSIT to ensure no damage occurred during sterilization, thereby preventing compromised units from entering critical process streams.
Single-Use Bioprocessing Systems: Disposable filtration components used in single-use bioprocessing platforms are integrity-tested directly within the system and subsequently discarded after a single lot or within 24 hours, supporting sterility assurance and eliminating cross-lot contamination risks.
Frequently Asked Questions on Filter Integrity Test
- Why is filter integrity testing important?
Filter integrity testing is essential because it validates filtration performance and supports regulatory compliance. It allows manufacturers to confirm sterility assurance levels, reduce contamination risks, and maintain consistent product quality across pharmaceutical, biotechnology, and food production processes. - What methods are used for filter integrity testing?
Common filter integrity testing methods include bubble point, diffusion, and water intrusion testing. These methods assess pore structure, airflow behavior, and potential leakage, enabling reliable verification of filter performance without damaging the filtration system or compromising sterile conditions. - When should a filter integrity test be performed?
A filter integrity test must be performed before and after filtration to confirm proper function. Pre-use testing ensures the filter is suitable for operation, while post-use testing verifies no breach occurred during processing, maintaining full compliance requirements. - What industries require filter integrity testing?
Filter integrity testing is required in pharmaceutical manufacturing, biotechnology production, medical devices, food and beverages, and sterile fill-finish operations. These industries depend on sterile filtration systems where regulatory standards mandate evidence of filter performance and contamination control. - Which industries contribute most to market demand?
The pharmaceutical and biotechnology sectors contribute the largest share of market demand. Increased adoption in food processing, medical device sterilization, and microelectronics also supports market expansion as organizations prioritize contamination prevention and validated filtration processes. - What regions are leading the filter integrity test market?
North America and Europe lead the market due to strong regulatory frameworks, advanced biomanufacturing infrastructure, and high investment in sterile processing. Rapid industrial growth in Asia-Pacific is driving significant additional demand from emerging pharmaceutical and biotechnology sectors. - What technologies are widely used in the market?
Automated integrity test instruments using diffusion, bubble point, and pressure-decay measurement technologies dominate the market. These systems enhance accuracy, data integrity, and throughput, aligning with industry requirements for validated sterility assurance and reliable filtration monitoring.
Conclusion
The filter integrity test market has been characterized by steady expansion, supported by growing regulatory expectations and rising demand for validated sterile processing. The dominance of bubble point and liquid-based testing reflects the emphasis on accuracy, sterility assurance, and compliance across critical applications.
Strong adoption within pharmaceutical and biopharmaceutical manufacturing continues to drive global revenue, while advances in automation and inline monitoring are improving operational efficiency. Increasing reliance on deterministic methods, single-use systems, and lifecycle-focused testing indicates a shift toward higher reliability and lower contamination risk. Overall, standardized integrity testing is strengthening product safety and enhancing quality-assurance practices worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



