Table of Contents
Overview
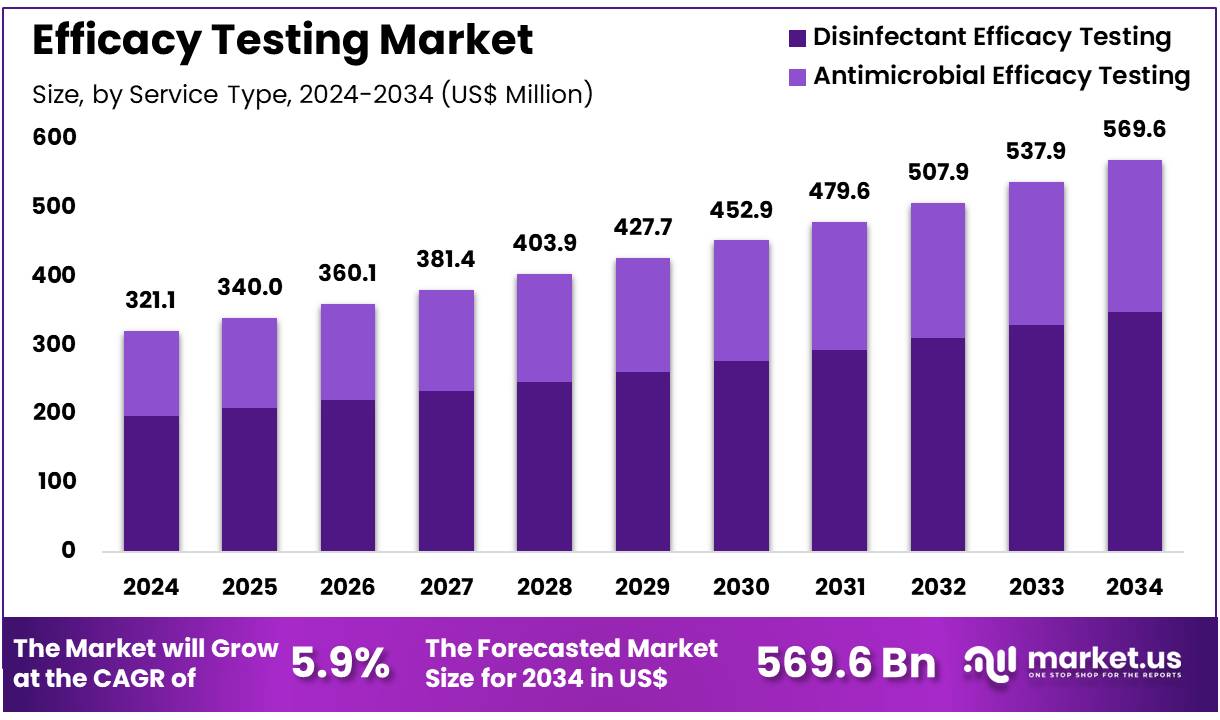
New York, NY – Nov 19, 2025 – Global Efficacy Testing Market size is expected to be worth around US$ 569.6 Million by 2034 from US$ 321.1 Million in 2024, growing at a CAGR of 5.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 44.8% share with a revenue of US$ 143.9 Million.
A structured framework for efficacy testing has been established to support companies in validating product performance with scientific precision. The formation of this framework has been designed to ensure that measurable outcomes, standardized procedures, and compliant data practices are consistently achieved across diverse product categories. The development of this approach has been driven by the increasing demand for verifiable evidence, regulatory alignment, and transparency in consumer product claims.
The framework includes controlled laboratory assessments, real-world application studies, and quantitative performance analyses. Each testing phase has been organized to capture objective metrics, enabling clear comparisons between baseline conditions and post-application results. The protocol includes rigorous sample selection, defined measurement intervals, and validated analytical tools to ensure accuracy. Statistical models are applied to determine significance levels, allowing findings to be communicated with confidence.
The adoption of this efficacy testing formation is expected to strengthen claim substantiation practices across sectors such as cosmetics, healthcare, food and beverage, and industrial solutions. The findings generated through this structure can be utilized in product development, regulatory submissions, marketing communication, and competitive benchmarking.
The introduction of this standardized formation is anticipated to support stakeholders in demonstrating product value, reducing uncertainty in market positioning, and increasing consumer trust. The initiative reflects the growing emphasis on evidence-based validation and the role of measurable performance outcomes in shaping modern product strategies.
Key Takeaways
- In 2024, the Efficacy Testing market generated revenue of US$ 321.1 million, supported by a 5.9% CAGR, and is projected to reach US$ 569.6 million by 2033.
- By service type, disinfectant efficacy testing dominated the market with a 61.3% share in 2024, surpassing antimicrobial efficacy testing.
- Based on application, pharmaceutical products accounted for the largest proportion, representing 52.5% of the market.
- North America emerged as the leading regional market, capturing a 44.8% share in 2024.
Regional Analysis
North America Leading the Efficacy Testing Market
North America accounted for the largest share of 44.8% in the Efficacy Testing Market, supported by rising regulatory vigilance, continuous progress in pharmaceutical and biotechnology fields, and an expanding requirement for quality assurance across consumer products. Data from the U.S. Food and Drug Administration (FDA) indicated that product recalls related to inefficacy and safety concerns increased by 15% between 2022 and 2023, which strengthened the need for more comprehensive testing frameworks.
The National Institutes of Health (NIH) recorded a 20% rise in funding allocated to clinical trials and efficacy-related studies in 2023, underscoring the region’s growing reliance on evidence-based product validation. Moreover, the Environmental Protection Agency (EPA) reported a 12% increase in demand for environmental efficacy testing in 2024, driven by tightened standards for disinfectants and pesticides.
The pharmaceutical sector demonstrated a notable 25% rise in outsourcing efficacy testing to specialized laboratories, as highlighted by the Pharmaceutical Research and Manufacturers of America (PhRMA). This trend has been reinforced by the wider adoption of technologies such as artificial intelligence and machine learning, which enhanced accuracy and operational efficiency in testing processes. These combined developments reflect the strong expansion of the efficacy testing industry across North America.
Asia Pacific Expected to Achieve the Highest CAGR
The Asia Pacific region is projected to record the fastest CAGR during the forecast period, driven by ongoing industrial growth, the rapid development of pharmaceutical and cosmetic industries, and increasing regulatory harmonization. According to the World Health Organization (WHO), clinical trial registrations in the region rose by 30% between 2022 and 2024, indicating a rising emphasis on product validation and safety compliance.
Regulatory authorities across major economies contributed to this momentum. The National Medical Products Administration (NMPA) in China reported a 22% increase in approvals for new drugs and medical devices in 2023, creating a strong demand for extensive efficacy testing. India’s Central Drugs Standard Control Organization (CDSCO) observed an 18% rise in requirements for bioequivalence and efficacy studies in 2024, influenced by the expansion of the country’s generic pharmaceutical sector.
Additionally, Japan’s Ministry of Health, Labour, and Welfare (MHLW) recorded a 15% increase in funding for quality control and testing infrastructure in 2023 to strengthen product reliability. These regulatory and industrial developments, combined with rising research and development investments, are expected to support accelerated market growth across the Asia Pacific region.
Emerging Trends
- Adoption of Complex Innovative Trial Designs: Regulatory support has increased for adaptive, Bayesian, and platform trial designs to enhance efficiency in efficacy evaluations. Under PDUFA VII (2022–2027), the FDA’s CID Review Program completed a public workshop by Q2 FY 2024 and is preparing draft guidance on Bayesian methods for release by the end of FY 2025.
- Stricter Vaccine Efficacy Thresholds: The World Health Organization now mandates that vaccine candidates demonstrate at least a 50% reduction in disease risk, with the lower 95% confidence interval exceeding 30%. These criteria raise performance requirements for efficacy assessment within global vaccine development programs.
- Expansion of Disinfectant Efficacy Validation: Updates to the U.S. EPA’s List N for SARS-CoV-2 disinfectants, current as of April 9, 2025, reflect continued evaluation of formulations. Hundreds of products have met the agency’s efficacy standards for viral reduction on hard, non-porous surfaces.
- Increased Funding and Publications in Bioequivalence Research: During FY 2024, the FDA’s GDUFA Regulatory Science and Research Program advanced bioequivalence methodologies, generating 77 peer-reviewed papers, 106 external posters, and 165 presentations. The program also issued 7 new grants and 6 contracts to support external research partnerships.
Use Cases
- Surface Disinfectant Validation: EPA’s List N protocol assesses disinfectant formulations against SARS-CoV-2, requiring products to achieve at least 99.9% viral reduction under labelled usage conditions. As of April 9, 2025, several hundred products have satisfied these efficacy benchmarks.
- Bioequivalence Testing for Complex Generics: In July 2024, the FDA approved the first generic bupivacaine liposome injectable suspension (1.3%), supported by GDUFA-funded research establishing in vitro to in vivo correlations. This marked a significant advancement in efficacy evaluation for complex liposomal generics.
- Adaptive Bioequivalence Designs for Highly Variable Drugs: CDER statisticians developed a master-scale average bioequivalence design using adaptive methods to address high within-subject variability. This approach can reduce sample size needs by up to 20% while sustaining statistical confidence in generic equivalence decisions.
- Vaccine Efficacy Endpoints in Phase 3 Trials: WHO’s COVID-19 vaccine tracker monitors global Phase 3 candidates using standardized primary endpoints that assess clinical disease reduction. This illustrates harmonized efficacy testing frameworks applied across a broad set of vaccine development programs.
Frequently Asked Questions on Efficacy Testing
- Why is efficacy testing important?
Efficacy testing is essential because it confirms that products meet their claimed benefits before market entry. It reduces product failure risks, strengthens regulatory compliance, and enhances consumer trust by providing objective, measurable evidence supporting safety, functionality, and performance expectations. - Which products require efficacy testing?
Products that require efficacy testing include cosmetics, personal care formulations, pharmaceuticals, disinfectants, medical devices, and nutraceuticals. These categories depend on validated performance data to ensure claims accuracy, safety assurance, and regulatory acceptance in domestic and international markets. - What methods are used in efficacy testing?
Methods used in efficacy testing include in-vitro assays, clinical trials, instrumental analysis, and consumer perception studies. These approaches quantify performance indicators, allowing manufacturers to demonstrate measurable outcomes that align with scientific standards, regulatory expectations, and product-specific claim requirements. - How long does efficacy testing take?
The duration of efficacy testing varies by product category and study complexity. Most evaluations range from several weeks to a few months, as controlled observations, data collection cycles, and statistical validation processes are required to confirm reliable and reproducible outcomes. - Who conducts efficacy testing?
Efficacy testing is conducted by accredited laboratories, research institutions, and specialized third-party testing agencies. These organizations follow standardized protocols, apply validated measurement techniques, and generate scientifically supported evidence required for regulatory submissions and substantiated marketing claims. - What is the efficacy testing market?
The efficacy testing market comprises service providers offering performance validation for cosmetics, pharmaceuticals, disinfectants, and consumer products. Demand is driven by stringent regulations, expanding product categories, and increased reliance on scientific evidence to support commercial claims and ensure compliance. - What factors are driving growth in this market?
Growth is driven by stricter regulatory frameworks, rising consumer awareness, expanded product innovation, and increased global emphasis on safety and claim substantiation. Market expansion is further supported by technological advancements that enable precise measurement of product performance. - Which industries contribute most to the efficacy testing market?
Industries contributing most include pharmaceuticals, cosmetics, personal care, household disinfectants, and medical devices. These sectors rely heavily on performance verification to meet regulatory approvals, strengthen competitive positioning, and support growing demand for scientifically validated product claims.
Conclusion
The global efficacy testing landscape is strengthening as industries prioritize validated performance, regulatory alignment, and transparent product claims. Market expansion is being supported by rising investments in clinical research, advanced trial designs, and stringent quality standards across regions. North America continues to lead due to strong regulatory oversight and technological integration, while Asia Pacific is poised for rapid growth driven by expanding pharmaceutical and cosmetic sectors.
Emerging trends such as adaptive trial models, stricter vaccine criteria, and enhanced bioequivalence research further reinforce the importance of evidence-based validation. Overall, efficacy testing is expected to remain a critical foundation for product reliability and market competitiveness.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



