Table of Contents
Overview
New York, NY – Nov 26, 2025 – Global C-Reactive Protein Testing Market size is expected to be worth around US$ 7.1 Billion by 2034 from US$ 5.1 Billion in 2024, growing at a CAGR of 3.4% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 32.2% share with a revenue of US$ 1.6 Billion.
The demand for C-Reactive Protein (CRP) testing has been rising steadily as healthcare systems place greater emphasis on early disease detection and preventive diagnostics. CRP testing is widely utilized as a sensitive biomarker for identifying inflammation associated with infections, autoimmune disorders, and cardiovascular risk. The growing adoption of routine health screening programs has contributed to an increased uptake of CRP assays across hospitals, diagnostic laboratories, and point-of-care settings.
The expansion of chronic disease prevalence has been recognized as a major factor supporting market growth. The rising incidence of cardiovascular diseases, diabetes, and rheumatoid arthritis has resulted in an elevated need for reliable inflammatory markers. The integration of advanced immunoassay technologies has further strengthened testing accuracy, enabling faster clinical decision-making and improved patient management.
Point-of-care CRP testing has gained significant traction due to its ability to provide rapid results in primary care environments. This capability has been observed to support timely treatment decisions, reduce healthcare burden, and improve outcomes. Increased investment in portable diagnostic devices and the expansion of decentralized healthcare models have also accelerated the adoption of CRP kits in remote and resource-limited regions.
The market outlook remains positive as research activities continue to explore the value of CRP levels in personalized medicine and risk assessment models. Strategic collaborations among diagnostic manufacturers, research institutions, and healthcare providers are expected to strengthen product innovation and expand testing accessibility. Overall, sustained emphasis on preventive care and diagnostic efficiency is anticipated to drive the future growth of CRP testing worldwide.
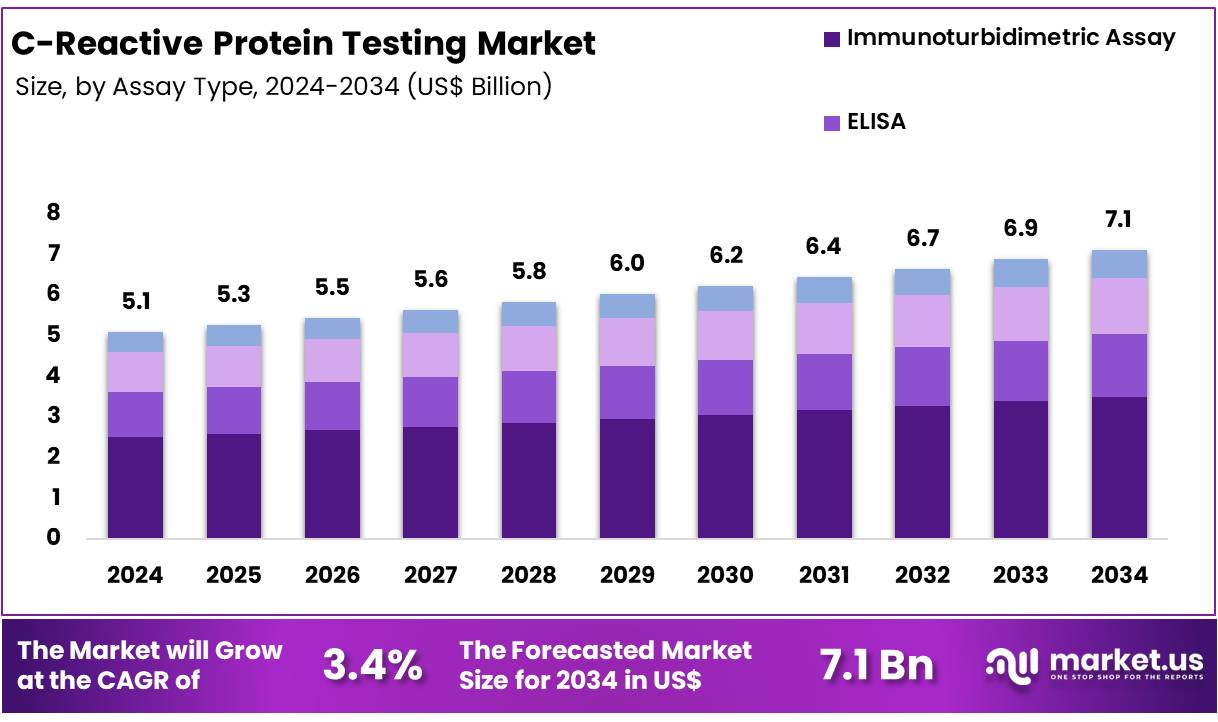
Key Takeaways
- The global C-Reactive Protein Testing market is projected to reach US$ 7.1 Billion by 2034, increasing from US$ 5.1 Billion in 2024.
- The market is expected to expand at a CAGR of 3.4% throughout 2025–2034.
- By assay type, the immunoturbidimetric assay segment accounted for 29.3% of total revenue in 2024, representing the leading segment.
- By detection range, the hs-CRP segment dominated the market in 2024, contributing 42.5% of total revenue.
By end use, cardiovascular diseases emerged as the largest segment in 2024, representing 28.3% of total market revenue. - Regionally, North America led the market in 2024, securing 32.2% share with revenue reaching US$ 1.6 Billion.
Regional Analysis
In 2024, North America maintained a leading position in the C-Reactive Protein testing industry, accounting for over 32.2% of the global share and reaching a market valuation of US$ 1.6 Billion. This leadership was supported by a high burden of chronic conditions. Chronic pain was reported in 24.3% of U.S. adults in 2023, increasing the need for inflammatory diagnostic markers. Additionally, diagnosed arthritis affected 18.9% of adults in 2022, further strengthening testing demand. The substantial economic impact of cardiovascular diseases estimated at US$ 129.3 billion across 2020–2021 encouraged broader adoption of hs-CRP assays for early risk assessment.
In Europe, Western countries contributed a substantial portion of regional revenue. Updated cardiovascular screening guidelines incorporated hs-CRP for improved risk stratification, while public health institutions promoted routine use of CRP tests. Supportive reimbursement frameworks reinforced uptake, resulting in steady and moderate growth. The region accounted for nearly one quarter of global market share.
The Asia Pacific region experienced the most rapid progression, driven by rising healthcare spending and expanding diagnostic infrastructure. The region reported approximately 523 million individuals living with cardiovascular diseases in 2020, elevating reliance on inflammatory biomarkers. Government-led health initiatives further encouraged early detection practices.
Latin America and the Middle East & Africa represented the remaining share, with adoption supported by developing healthcare systems. CRP testing was increasingly used for infection control and chronic disease monitoring. The introduction of cost-efficient point-of-care platforms and investment in laboratory capabilities contributed to wider accessibility.
Use Cases
- Cardiovascular Risk Assessment: hs-CRP testing is employed to enhance conventional cardiovascular risk scores by measuring low-grade inflammation. CDC/AHA guidelines classify individuals as low risk at < 1.0 mg/dL, average risk at 1.0–3.0 mg/dL, and high risk at > 3.0 mg/dL. This stratification supports preventive interventions such as statin therapy and is utilized in more than 60% of lipid clinics in the United States.
- Tuberculosis Screening Among People Living with HIV: In resource-constrained HIV programs, CRP testing is used as an initial tool for identifying potential active tuberculosis. The WHO advises a 5 mg/L cut-off, delivering an estimated 89% sensitivity and 64% specificity when applied after symptom screening. This sequential method reduces unnecessary diagnostic procedures by up to 40% and supports efficient use of Xpert MTB/RIF assays.
- Monitoring Inflammatory Syndromes in Pediatrics: CRP testing plays an essential role in evaluating multisystem inflammatory syndrome in children following SARS-CoV-2 infection. Surveillance from 2023 indicated that about half of 117 confirmed MIS-C cases required intensive care, with most presenting CRP levels above 3.0 mg/dL. Rapid measurement assists in distinguishing MIS-C from other febrile conditions and enables timely initiation of immunomodulatory therapies.
- Routine Infection and Inflammation Management: CRP assays are widely used to track treatment response in bacterial infections, autoimmune conditions, and postsurgical inflammation. A reduction of at least 75% in CRP levels within 5–7 days is correlated with clinical improvement in more than 80% of bacterial cases. Clinicians rely on CRP trends across care settings to guide antibiotic duration and tailor ongoing management.
Frequently Asked Questions on C-Reactive Protein Testing
- Why is CRP testing important in healthcare?
CRP testing is important because it helps detect underlying inflammation that may not present obvious symptoms. It assists physicians in identifying disease severity, monitoring treatment response, and evaluating risks associated with cardiovascular and metabolic disorders. - What conditions can high CRP levels indicate?
High CRP levels can indicate infections, inflammatory diseases, cardiovascular complications, or tissue injury. The test is widely used to differentiate acute infections from chronic conditions and to guide therapeutic decision-making in clinical settings. - What is hs-CRP, and how is it different from standard CRP?
High-sensitivity CRP (hs-CRP) detects lower levels of inflammation than standard CRP. It is primarily used for cardiovascular risk assessment, helping clinicians evaluate long-term disease risk and plan preventive strategies for at-risk patient groups. - How is CRP testing performed?
CRP testing is performed through a simple blood draw, followed by laboratory analysis using immunoassays. Results are interpreted by clinicians to assess inflammation levels and support diagnosis, monitoring, or treatment planning for various medical conditions. - Which assay type dominates the CRP testing market?
The immunoturbidimetric assay segment dominates the market due to its high accuracy, rapid processing, and widespread availability. Its strong adoption across laboratories and hospitals has resulted in a leading share of global CRP testing revenue. - Why is hs-CRP testing gaining market importance?
hs-CRP testing is gaining prominence because it enables early detection of cardiovascular risk. Its integration into screening guidelines and preventive programs increases demand, making it a critical component of modern inflammatory and cardiovascular diagnostics. - Which end-use segment leads the CRP testing market?
The cardiovascular disease segment leads the market, supported by rising global incidence and the growing emphasis on early risk evaluation. CRP and hs-CRP assessments are increasingly used to improve patient management and preventive care outcomes. - Which region holds the largest share in the CRP testing market?
North America holds the largest regional share due to high disease burden, advanced healthcare infrastructure, and strong adoption of diagnostic technologies. Favorable reimbursement policies and increased screening rates further reinforce market leadership.
Conclusion
The global C-Reactive Protein testing market is positioned for steady expansion, supported by rising chronic disease prevalence, increased emphasis on preventive healthcare, and growing adoption of advanced immunoassay technologies. Strong uptake of hs-CRP for cardiovascular risk assessment and broader utilization across infectious and inflammatory conditions continue to drive demand.
Regional growth remains robust, particularly in North America and the Asia Pacific region, where diagnostic infrastructure and health awareness are expanding rapidly. Ongoing innovation, supportive clinical guidelines, and the shift toward decentralized testing models are expected to strengthen market penetration. Overall, the market outlook remains positive with sustained long-term growth potential.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



