Table of Contents
Overview
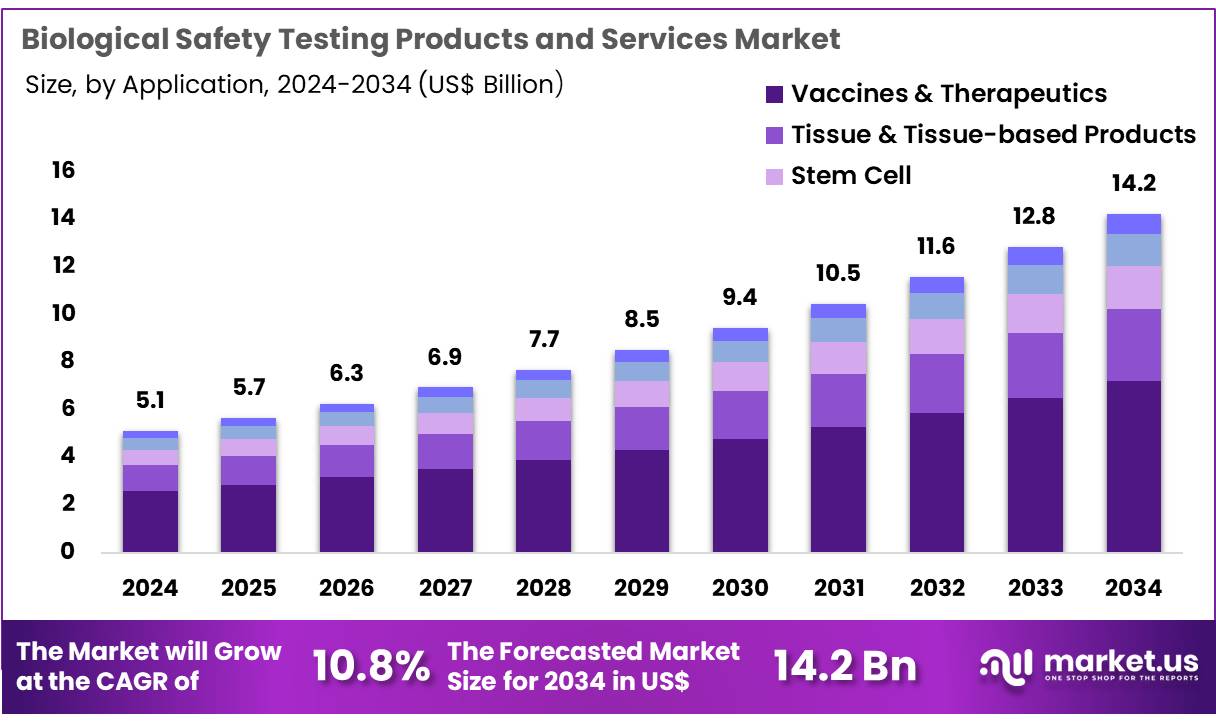
New York, NY – Nov 28, 2025 – Global Biological Safety Testing Products and Services Market size is expected to be worth around US$ 14.2 billion by 2034 from US$ 5.1 billion in 2024, growing at a CAGR of 10.8% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 38.3% share with a revenue of US$ 2.0 Billion.
The global market for Biological Safety Testing Products and Services has been experiencing steady expansion, driven by the rising volume of biopharmaceutical research, stringent regulatory frameworks, and the growing emphasis on product quality assurance. Increasing adoption of biologics, vaccines, cell and gene therapies has created substantial demand for advanced safety assessment tools. As a result, a broad portfolio of assays, instruments, and services has been introduced to support contamination detection, sterility verification, and viral safety evaluation.
The market growth has been supported by the escalating prevalence of chronic and infectious diseases, which has increased investment in drug development pipelines. The rise in outsourcing activities toward specialized contract research and testing organizations has further strengthened market performance. Biologics manufacturing facilities are increasingly relying on third-party expertise to comply with global regulatory standards, including those set by the FDA, EMA, and other international authorities.
Continuous technological innovations, such as rapid microbiological methods, automation, and high-sensitivity analytical platforms, have improved testing efficiency and accuracy. Demand for endotoxin testing, sterility testing, and mycoplasma testing remains particularly prominent. North America is maintaining a leading position due to the presence of advanced biopharmaceutical manufacturing infrastructure, while Asia-Pacific is recording notable growth supported by expanding R&D investments.
The market outlook remains positive, as the expansion of biopharmaceutical production, along with increasing regulatory vigilance, continues to support adoption of biological safety testing solutions across global laboratories and manufacturing units.
Key Takeaways
- In 2024, the biological safety testing products and services market generated US$ 5.1 billion in revenue, growing at a CAGR of 10.8%, and is projected to reach US$ 14.2 billion by 2033.
- The product type segment comprises reagents & kits, services, and instruments, with reagents & kits leading the market in 2024 with a 45.3% share.
- By application, the market is categorized into vaccines & therapeutics, tissue & tissue-based products, stem cell, gene therapy, and blood & blood-based products. Vaccines & therapeutics accounted for the largest portion, representing 50.6% of the market.
- In terms of test type, the market includes endotoxin tests, bioburden tests, cell line authentication & characterization tests, sterility tests, and others. Endotoxin tests emerged as the dominant segment with a 40.9% revenue share.
- North America led the global market in 2024, securing a 38.3% share.
Regional Analysis
North America Leading the Biological Safety Testing Products and Services Market
North America accounted for the largest revenue share of 38.3% in the biological safety testing products and services market, supported by a stringent regulatory environment and the substantial scale of biopharmaceutical development and manufacturing activities in the region. The U.S. Food and Drug Administration (FDA) continues to implement rigorous safety and sterility guidelines, prompting biopharmaceutical manufacturers to adopt more advanced testing technologies.
Regulatory updates issued in 2023 regarding sterile drug products and microbial contamination prevention further strengthened compliance requirements. In addition, sustained investment in biological research has reinforced market growth. The NIH Data Book indicates that in Fiscal Year 2023, the National Institutes of Health allocated US$ 34.92 billion in extramural research funding across 58,951 awards, supporting extensive innovation in biologics, including cell and gene therapies. This strong research infrastructure has continued to drive regional demand for comprehensive safety testing solutions in 2024.
Asia Pacific Expected to Record the Fastest CAGR
Asia Pacific is projected to witness the highest CAGR during the forecast period, driven by rapid expansion of the biopharmaceutical sector and growing healthcare investments. China and India, in particular, are expected to strengthen their biomanufacturing capabilities, increasing the requirement for extensive testing services to adhere to global quality benchmarks.
Government initiatives, such as India’s “Make in India” program, are promoting domestic pharmaceutical production and are anticipated to elevate the adoption of stringent safety testing practices. Rising incidences of infectious diseases across the region are also contributing to heightened demand for diagnostic and safety testing solutions.
Evolving regulatory frameworks increasingly aligned with international standards, coupled with expanding R&D activity, are expected to support strong and sustained market growth in Asia Pacific over the coming years.
Emerging Trends
Integration of Next-Generation Sequencing for Rapid Pathogen Detection
The integration of next-generation sequencing with advanced molecular detection programs is enabling faster pathogen characterization across viruses and bacteria. This combined approach enhances outbreak investigations and supports comprehensive analysis of infectious diseases within U.S. public health laboratory networks.
Expansion of the Laboratory Response Network (LRN-B)
The continued expansion of the CDC’s LRN-B to nearly 120 laboratories demonstrates a shift toward distributed biosafety capabilities. These facilities span state, local, military, veterinary, and international locations, strengthening national capacity for specialized testing of high-risk environmental and clinical samples.
Regulatory Emphasis on Safety Testing for Cellular and Gene Therapies
Recent draft guidances issued by the FDA’s Center for Biologics Evaluation and Research outline detailed sterility and adventitious agent testing requirements for advanced therapies. These documents reflect rising regulatory oversight as the pipeline for cellular and gene therapies continues to expand.
Adoption of Portable Sequencing Technologies
Portable nanopore sequencing platforms are being increasingly adopted for field-based biosafety testing. Their stability across varied environmental conditions supports decentralized pathogen surveillance and expands testing capabilities beyond conventional laboratory environments.
Shift Toward Protocol-Driven, Risk-Based Biorisk Management
The updated sixth edition of the CDC’s Biosafety in Microbiological and Biomedical Laboratories emphasizes structured, protocol-driven risk assessments. New guidance on large-scale biosafety and laboratory sustainability demonstrates a broader industry transition toward formalized, risk-based management practices.
Frequently Asked Questions on Biological Safety Testing Products and Services
- Why is biological safety testing important?
Biological safety testing is essential because it confirms the absence of contaminants such as viruses, mycoplasma, endotoxins, and residual host-cell impurities. The testing ensures that biopharmaceutical products meet global quality expectations, safeguarding patient safety and supporting regulatory approvals. - Which industries use biological safety testing services?
Biopharmaceutical companies, contract research organizations, academic laboratories, vaccine manufacturers, and cell-therapy developers are major adopters. These industries rely on safety testing to validate product integrity, meet stringent regulatory requirements, and maintain continuous product quality throughout development and commercialization. - What are the key types of biological safety tests performed?
Common testing types include sterility testing, mycoplasma detection, endotoxin quantification, bioburden assessment, viral safety assays, and cell-line characterization. These tests collectively evaluate microbial contamination, genetic stability, and product consistency across different stages of biologics manufacturing. - Who regulates biological safety testing activities?
Regulatory oversight is provided by agencies such as the FDA, EMA, WHO, and ICH. These bodies establish standardized testing guidelines that biopharmaceutical manufacturers must follow to ensure product safety, batch quality, and compliance with global regulatory frameworks. - Which regions dominate the biological safety testing market?
North America leads due to strong biopharmaceutical manufacturing capacity, established regulatory frameworks, and widespread adoption of advanced testing technologies. Europe follows, while Asia-Pacific is emerging rapidly because of expanding biotech investments and growing pharmaceutical outsourcing activities. - What technologies are commonly used in the market?
Technologies such as PCR-based assays, next-generation sequencing, rapid microbial detection systems, immunoassays, and chromatography platforms are widely utilized. These tools enable high-precision contaminant detection, improved workflow efficiency, and faster decision-making in biologics development. - How is outsourcing influencing market growth?
Outsourcing to specialized contract testing laboratories is increasing because biopharmaceutical companies seek cost efficiency, regulatory expertise, and rapid turnaround times. This shift enhances operational flexibility, reduces capital expenditure, and accelerates development timelines for complex biologics.
Conclusion
The biological safety testing products and services market is expected to maintain a strong growth trajectory, supported by expanding biopharmaceutical research, rising production of advanced therapies, and stricter global regulatory standards. Increasing reliance on specialized testing laboratories, ongoing technological advancements, and heightened focus on contamination control continue to strengthen market adoption.
North America is projected to retain its leadership position, while Asia-Pacific is anticipated to record accelerated growth due to expanding manufacturing capabilities. Overall, sustained investment in biologics, vaccines, and cell and gene therapies is expected to reinforce demand for comprehensive safety testing solutions across global healthcare and research environments.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



