Table of Contents
Overview
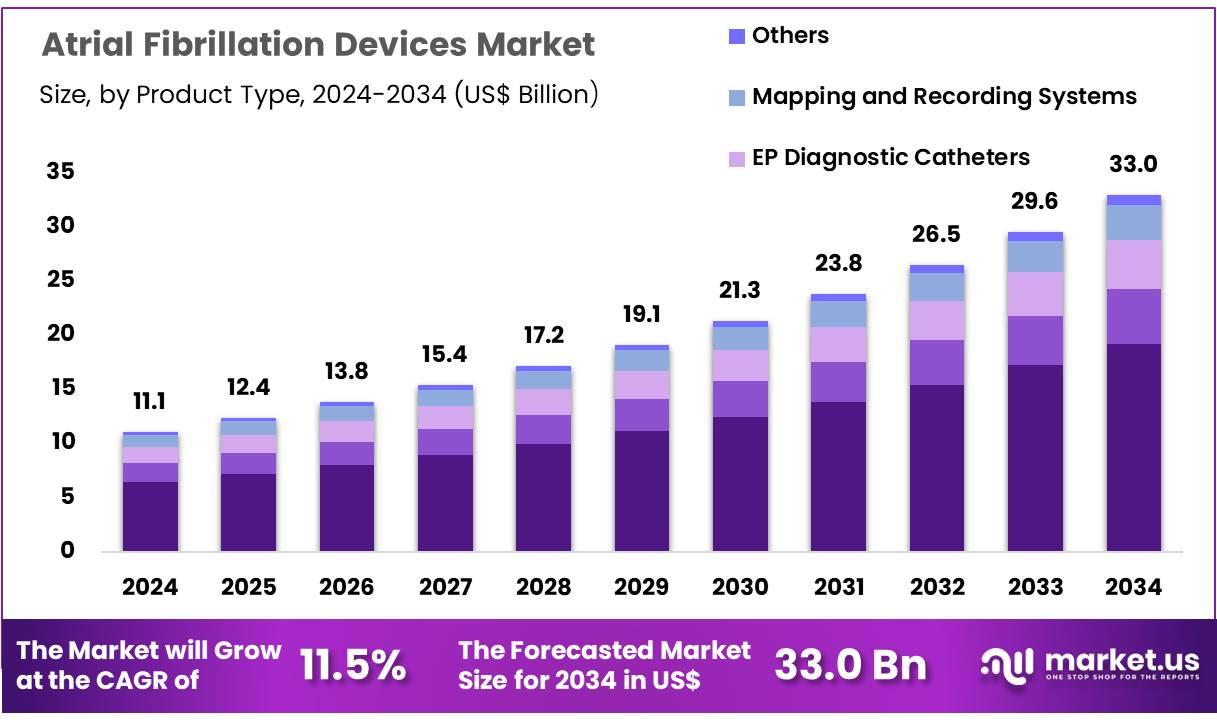
New York, NY – Nov 27, 2025 – Global Atrial Fibrillation Devices Market size is expected to be worth around US$ 33.0 billion by 2034 from US$ 11.1 billion in 2024, growing at a CAGR of 11.5% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 42.2% share with a revenue of US$ 4.7 Billion.
The global market for atrial fibrillation (AF) devices is witnessing steady expansion, driven by the increasing prevalence of cardiovascular disorders and the growing adoption of minimally invasive treatment solutions. Significant demand has been observed for advanced diagnostic and therapeutic systems, including ablation catheters, mapping systems, implantable monitors, and cardiac rhythm management devices. The rising burden of AF, which affects millions of individuals worldwide, has led to enhanced investments in innovative technologies that enable precise monitoring and efficient rhythm control.
Market growth has been supported by continuous advancements in catheter ablation techniques, improved device safety profiles, and wider acceptance of implantable cardiac monitors. The expansion of electrophysiology laboratories and the escalation of screening programs have also contributed to higher adoption rates. In addition, supportive reimbursement policies across key markets have strengthened the commercial viability of AF intervention procedures.
North America continues to maintain a dominant position due to established healthcare infrastructure and early technology adoption, while the Asia-Pacific region is exhibiting strong potential owing to large patient populations and expanding healthcare access. Strategic collaborations between device manufacturers, hospitals, and research organizations are expected to accelerate product development and broaden clinical applications.
Key Takeaways
- The atrial fibrillation devices market generated US$ 11.1 billion in revenue in 2024, advancing at a CAGR of 11.5%, and is projected to achieve US$ 33.0 billion by 2033.
- The product landscape includes EP ablation catheters, cardiac monitors or implantable loop recorders, EP diagnostic catheters, mapping and recording systems, and other devices, with EP ablation catheters dominating the segment in 2024 with a 58.2% share.
- Based on end users, the market is categorized into hospitals & cardiac centers, ambulatory surgical centers, and others, with hospitals & cardiac centers accounting for 62.3% of the market in 2024.
- North America remained the leading regional market, capturing 42.2% of the total share in 2024.
Regional Analysis
North America is Leading the Atrial Fibrillation Devices Market
North America accounted for the largest revenue share of 42.2%, supported by the high prevalence of atrial fibrillation and the presence of a well-developed healthcare system. The Centers for Disease Control and Prevention reported in 2023 that an estimated 12.1 million adults in the United States are expected to be affected by atrial fibrillation by 2030, indicating the substantial patient pool requiring continuous management.
The region benefits from a strong network of electrophysiology (EP) laboratories, where a considerable number of ablation procedures are undertaken each year. According to the American College of Cardiology, more than 200,000 catheter ablation procedures were performed in the U.S. in 2022, reflecting the high procedural volume. Favorable reimbursement structures and continuous technological advancements further reinforce the region’s leadership position.
In addition, ongoing research and development activities, supported by multiple FDA approvals for novel ablation technologies since 2021, and the presence of major medical device manufacturers contribute to sustained market expansion. Growing adoption of advanced cardiac monitoring technologies, including implantable loop recorders for early AFib detection, also strengthens demand.
Asia Pacific Is Expected to Register the Highest CAGR
Asia Pacific is projected to record the fastest growth during the forecast period, driven by rising awareness of atrial fibrillation and increasing investments in healthcare infrastructure. The region’s aging population, particularly in China and Japan, continues to elevate AFib prevalence. A 2021 study in the *Journal of the American Heart Association* estimated over 10 million AFib cases in China alone, reflecting a significant treatment need.
Expanding cardiac care capacity is further supporting adoption, with more than 50 new EP laboratories added in China between 2021 and 2023, as reported in national health statistics. Government initiatives aimed at improving access to cardiac care such as India’s expansion of cardiology services in Tier-II and Tier-III cities are enhancing treatment availability.
The growing use of remote cardiac monitoring technologies in countries such as Australia is also contributing to the region’s rapid market growth.
Use Case
- At-Home Population Screening: Wearable smartwatches integrated with AF detection algorithms have been utilized for large-scale population screening. Evidence from the Fitbit Heart Study, involving more than 450,000 participants, demonstrated that Irregular Rhythm Notifications achieved 98% accuracy, enabling early clinical evaluation in asymptomatic users.
- Post-Stroke Arrhythmia Surveillance: Extended external loop recorders have been applied to identify atrial fibrillation in cases of cryptogenic stroke. A pivotal trial showed that 30-day monitoring detected AF in 16.1% of patients, compared with 3.3% using repeated 24-hour Holter monitoring, supporting earlier anticoagulation therapy to reduce recurrence.
- Extended Holter Monitoring in Cryptogenic Stroke Survivors: Seven-day Holter monitoring conducted after cryptogenic stroke detected AF in 9% of patients at baseline. Continued rhythm assessment across a 36-month period increased cumulative AF detection to 36%, assisting long-term management strategies and anticoagulation planning in high-risk populations.
- Prescription-Based Remote ECG Monitoring: Prescription-grade wearable ECG systems, such as the Verily Study Watch equipped with an Irregular Pulse Monitor, are used by adults diagnosed with or predisposed to AF. Daily ECG recordings are transmitted remotely to clinicians, supporting timely therapeutic decisions and individualized treatment pathways.
Frequently Asked Questions on Atrial Fibrillation Devices
- What types of devices are used for atrial fibrillation management?
The primary device types include diagnostic ECG monitors, implantable loop recorders, cardiac ablation catheters, and pacemakers. These technologies facilitate continuous monitoring, precise mapping, and controlled rhythm correction in patients with varying severity of atrial fibrillation. - What role do implantable monitors play in atrial fibrillation?
Implantable monitors deliver long-term rhythm surveillance that enables detection of asymptomatic episodes. Their continuous data collection supports earlier clinical intervention, improved treatment planning, and enhanced management of stroke risk associated with atrial fibrillation. - What are the benefits of atrial fibrillation devices?
These devices enhance diagnostic accuracy, support personalized treatment strategies, and contribute to reduced hospitalization rates. Their use enables improved rhythm control, earlier identification of disease progression, and better long-term cardiovascular health outcomes. - How is atrial fibrillation diagnosed using these devices?
Diagnosis is achieved through continuous or episodic rhythm monitoring using ECG devices, Holter monitors, or implantable loop recorders. These tools capture irregular electrical patterns that allow precise identification of atrial fibrillation episodes. - What advancements are being seen in atrial fibrillation device technology?
Advancements include improved mapping systems, minimally invasive ablation tools, AI-enabled diagnostic platforms, and compact implantable monitors. These innovations support earlier detection, better procedural outcomes, and more efficient long-term rhythm management. - Which region leads the atrial fibrillation devices market?
North America leads due to advanced healthcare infrastructure, higher diagnosis rates, and strong adoption of innovative electrophysiology technologies. Europe follows closely, supported by expanding clinical programs and increasing investment in cardiac rhythm management solutions. - How is technology innovation influencing the market?
Innovation in mapping systems, energy-delivery platforms, and long-term monitoring devices continues to enhance procedural accuracy and patient outcomes. These advancements support wider clinical acceptance and contribute to sustained demand across global markets. - What impact does the growing elderly population have on the market?
The elderly population exhibits higher atrial fibrillation incidence, creating strong demand for diagnostic and therapeutic devices. This demographic trend supports long-term market
Conclusion
The atrial fibrillation devices market is expected to demonstrate sustained expansion, supported by rising disease prevalence, technological innovation, and increasing adoption of minimally invasive treatment modalities. Strong demand for advanced diagnostic and therapeutic systems, coupled with expanding electrophysiology capacity, continues to reinforce market growth across major regions.
North America remains a dominant contributor, while Asia-Pacific is set to achieve significant momentum due to improving healthcare access and large patient populations. Ongoing advancements in ablation technologies, remote monitoring solutions, and implantable devices are anticipated to enhance clinical outcomes, strengthen early detection capabilities, and drive long-term market advancement globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



