Table of Contents
Overview
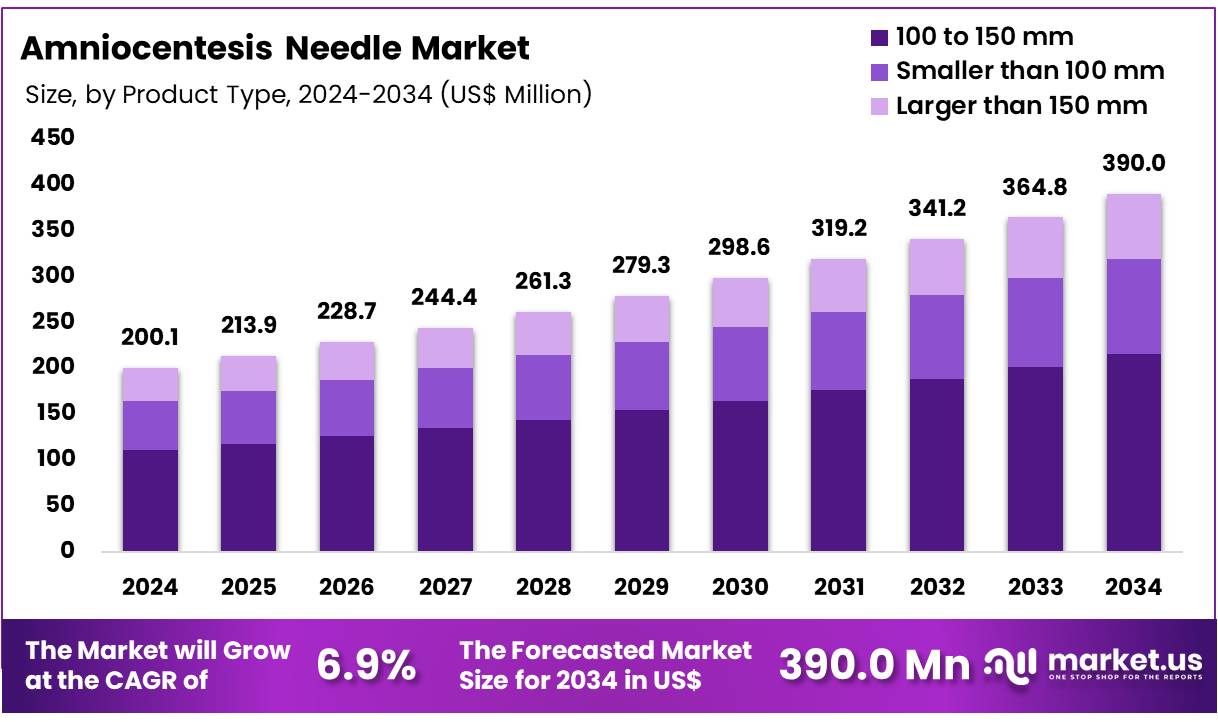
New York, NY – Nov 25, 2025 – The Global Amniocentesis Needle Market size is expected to be worth around US$ 390.0 Million by 2034 from US$ 200.1 Million in 2024, growing at a CAGR of 6.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 42.2% share with a revenue of US$ 84.4 Million.
The development of the Amniocentesis Needle has been driven by the growing emphasis on safe and accurate prenatal diagnostic procedures. The device has been designed to support clinicians in obtaining high-quality amniotic fluid samples while minimizing patient discomfort and procedural risk. The basic formation of the needle reflects a combination of precision engineering and patient-centric design, which has contributed to its increasing adoption across healthcare facilities.
The needle is generally composed of a thin, hollow stainless-steel shaft that allows smooth insertion through maternal tissue. Its structure is characterized by a sharp, beveled tip that facilitates controlled penetration, while an outer guide needle is often incorporated to enhance stability during the procedure. A stylet is positioned within the needle to maintain rigidity prior to fluid aspiration, and it is withdrawn once the correct placement within the amniotic sac has been confirmed. The hub is ergonomically designed to support easy handling and secure attachment to standard syringes.
The formation of the Amniocentesis Needle has been optimized to reduce procedural complications, including maternal bleeding or fetal injury. The use of advanced biocompatible materials has strengthened durability and improved compatibility with ultrasound guidance. The increasing demand for reliable prenatal testing has supported continuous product refinement, and ongoing innovation is expected to enhance safety and accuracy further. The needle’s engineering reflects the industry’s commitment to improving diagnostic outcomes. The steady rise in prenatal screenings and the need for precise sampling methods have positioned the Amniocentesis Needle as an essential tool in modern obstetrics.
Key Takeaways
- The global amniocentesis needle market generated US$ 200.1 million in 2024, supported by a CAGR of 6.9%, and the market size is projected to reach US$ 390.0 million by 2033.
- By product type, the market is segmented into 100–150 mm, smaller than 100 mm, and larger than 150 mm needles, with the 100–150 mm category accounting for 55.2% of the market share in 2024.
- Based on application, the market covers amniocentesis procedures, fetal blood transfusion, cordocentesis procedures, amnioreduction procedures, and amnioinfusion procedures, with amniocentesis procedures representing 59.3% of total demand.
- In terms of end users, the market is classified into hospitals & clinics, diagnostic centers, and others, where hospitals & clinics dominated the market with a 61.2% revenue share in 2024.
- Regionally, North America remained the leading market, contributing 42.2% of global revenue in 2024.
Regional Analysis
North America is Leading the Amniocentesis Needle Market
North America accounted for the largest revenue share of 42.2%, supported by the rising volume of prenatal diagnostic procedures, the growing proportion of pregnancies among women aged 35 years and above, and continuous advancements in genetic testing technologies. Data from the Centers for Disease Control and Prevention (CDC) indicated a 2.3% increase in births among women aged 35 and older in 2023, contributing to higher demand for prenatal diagnostic tools. The National Institutes of Health (NIH) reported a 12% rise in federal funding for fetal medicine research in 2023, which strengthened innovation in amniocentesis needle design and safety.
Hospitals and clinics in the region increasingly adopted advanced needle systems, supported by FDA-approved products launched by leading manufacturers such as Becton Dickinson and CooperSurgical. Updated recommendations issued by the American College of Obstetricians and Gynecologists (ACOG) in late 2023 emphasized the use of genetic amniocentesis for high-risk pregnancies, further driving market expansion. Additionally, the Canadian Institute for Health Information (CIHI) recorded an increase in prenatal diagnostic procedures during 2023, reinforcing regional market growth.
Asia Pacific is Expected to Register the Fastest CAGR
The Asia Pacific region is anticipated to witness the highest growth rate, driven by rapid improvements in healthcare infrastructure, rising awareness of genetic disorders, and government programs supporting prenatal care. India’s Ministry of Health and Family Welfare documented a notable rise in prenatal screening centers in 2023, directly boosting the uptake of amniocentesis equipment.
Stricter prenatal testing regulations implemented by China’s National Health Commission (NHC) in 2022 resulted in an expansion of approved diagnostic facilities by 2024. Japan’s Society of Obstetrics and Gynecology reported continuous growth in advanced prenatal procedures since 2022, supported by an aging maternal population. Key manufacturers such as Medtronic and Rocket Medical are expected to strengthen distribution networks across emerging markets, while Australia’s 2023 investments in genetic research are projected to enhance access to amniocentesis procedures, supporting overall regional market growth.
Use Cases
- Genetic and chromosomal diagnosis: Amniocentesis is widely used for detecting genetic and chromosomal abnormalities. In a public health registry involving 1,040 women undergoing mid-trimester procedures, fetal abnormalities were identified in 4.3% of cases, and the diagnostic accuracy reached a highly reliable 99.4%.
- Definitive birth-defect confirmation: The procedure provides a dependable method for confirming congenital disorders, offering nearly 99% accuracy in identifying major genetic and chromosomal conditions. Laboratory results are generally available within 10 to 14 days, supporting timely clinical decision-making.
- Advanced maternal-age screening: Historical records show that in 1990, around 40% of pregnant women aged 35 years or older opted for amniocentesis or chorionic villus sampling. This reflects its long-established role in evaluating pregnancy-related risks among older expectant mothers.
- Patient choice and uptake: Current estimates indicate that approximately 5–10% of pregnant women choose to undergo amniocentesis annually. This consistent uptake highlights its continued acceptance, perceived clinical value, and importance within routine prenatal care pathways.
Frequently Asked Questions on Amniocentesis Needle
- How is an amniocentesis needle used?
The needle is inserted through the abdominal wall under ultrasound guidance to reach the amniotic sac. Once positioned correctly, fluid is aspirated for genetic, biochemical, or chromosomal analysis to support fetal health assessment. - What sizes are available for amniocentesis needles?
Amniocentesis needles are available in multiple lengths, commonly under 100 mm, between 100–150 mm, and above 150 mm. The 100–150 mm segment is widely used due to its balance of maneuverability and clinical accuracy. - Are amniocentesis needles safe for mother and fetus?
The needle is engineered to minimize complications through sharp precision tips, biocompatible materials, and enhanced ultrasound visibility. When performed by trained professionals, the procedure demonstrates a low risk profile and high diagnostic reliability. - What is driving the growth of the amniocentesis needle market?
Market growth is driven by rising prenatal diagnostic procedures, increasing maternal age, greater awareness of genetic disorders, and improved healthcare infrastructure. Technological advancements and regulatory support further strengthen global market expansion. - Which product segment holds the largest market share?
The 100–150 mm needle category holds the largest share due to its suitability for a wide range of prenatal procedures. Its widespread acceptance reflects clinical preference for accuracy, safety, and compatibility with established diagnostic protocols. - Which application dominates the amniocentesis needle market?
Amniocentesis procedures represent the dominant application segment, driven by rising demand for genetic testing and fetal health assessments. The procedure’s role in detecting chromosomal abnormalities significantly contributes to its leading market position. - Which end-user segment leads the market?
Hospitals and clinics account for the largest revenue share owing to their advanced diagnostic facilities, specialized maternal care units, and higher patient footfall. Their adoption of improved needle technologies enhances procedural efficiency and safety. - 9. Which region currently leads the market?
North America leads the global market due to high prenatal screening rates, technological advancements, and strong healthcare infrastructure. Increased maternal age and supportive clinical guidelines contribute significantly to regional market dominance.
Conclusion
The global amniocentesis needle market is positioned for steady expansion, supported by rising prenatal diagnostic procedures, increasing maternal age, and advancements in genetic testing. Continued improvements in needle design, biocompatible materials, and ultrasound-guided precision have strengthened clinical adoption.
Demand from hospitals and clinics remains dominant, while North America leads due to strong healthcare infrastructure. Meanwhile, Asia Pacific is expected to register the fastest growth, driven by expanding prenatal programs and improved access to diagnostic services. Overall, the market is expected to grow consistently as healthcare systems emphasize accurate, reliable, and safe prenatal evaluation methods.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



