Table of Contents
Overview
New York, NY – Sep 22, 2025 – Global Gene Therapy in CNS Disorder Market size is expected to be worth around US$ 47.9 Billion by 2033 from US$ 10 Billion in 2024, growing at a CAGR of 19.01% during the forecast period from 2025 to 2033. With a market share over 38.30% North America held a strong lead in 2023, reaching US$ 3.2 Billion in revenue.
The field of gene therapy for central nervous system (CNS) disorders is witnessing rapid advancements, marking a transformative phase in neurological medicine. The growing prevalence of neurodegenerative and genetic disorders, coupled with the limited effectiveness of conventional treatment approaches, has accelerated the demand for innovative therapies. Gene therapy offers the potential to address the underlying genetic causes of diseases such as Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA), rather than merely alleviating symptoms.
The market for CNS-targeted gene therapy is being driven by technological progress in viral vectors, genome editing tools, and delivery mechanisms capable of crossing the blood–brain barrier. Strategic collaborations between pharmaceutical companies, biotechnology firms, and academic research institutions are fostering breakthroughs and expediting clinical development. Several late-stage clinical trials are currently evaluating the efficacy and safety of gene therapy candidates, underscoring the strong pipeline in this therapeutic area.
Regulatory support, in the form of orphan drug designations and fast-track approvals, is contributing to accelerated commercialization prospects. Despite challenges such as high treatment costs, complex manufacturing processes, and long-term safety concerns, the outlook remains cautiously optimistic. Investment momentum, coupled with strong patient advocacy, is expected to expand access and drive future adoption.
Gene therapy in CNS disorders is positioned as a paradigm shift in modern medicine, with the potential to deliver long-lasting therapeutic benefits and transform patient outcomes globally.
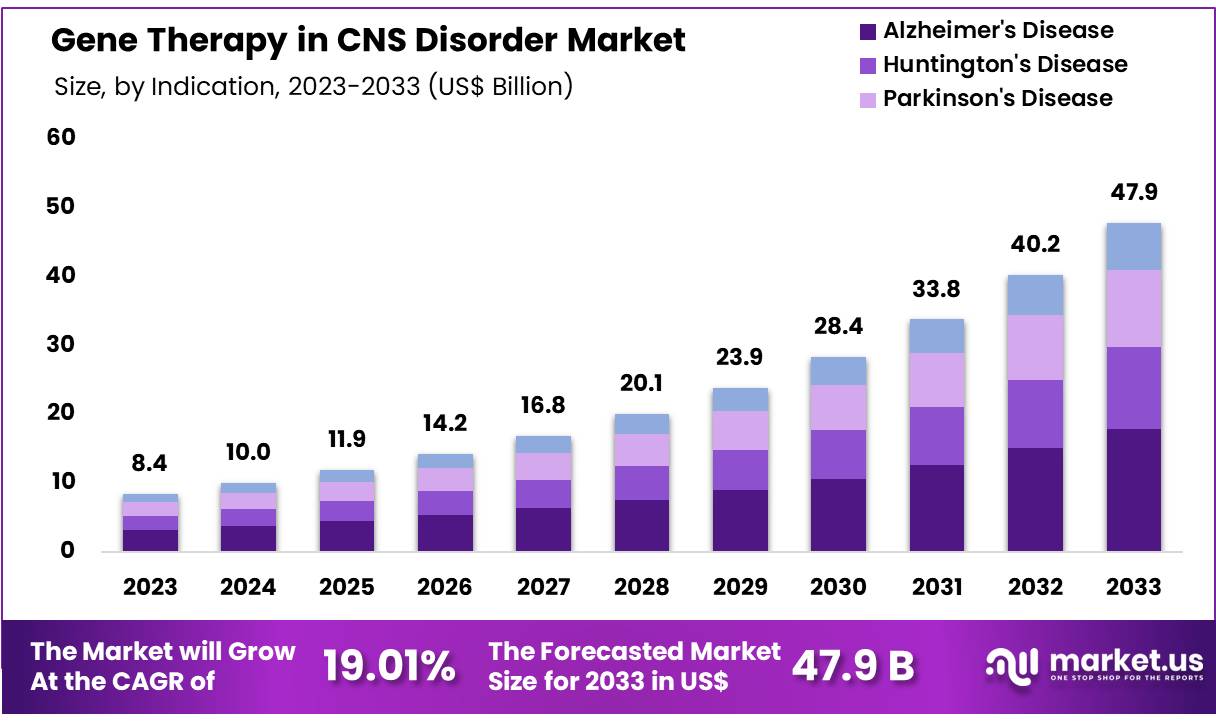
Key Takeaways
- Market Size: The global gene therapy in CNS disorder market is projected to reach USD 47.9 billion by 2033, rising from USD 8.4 billion in 2023.
- Market Growth: The industry is anticipated to expand at a compound annual growth rate (CAGR) of 19.01% between 2024 and 2033.
- Indication Analysis: By indication, Alzheimer’s disease dominated the market in 2023, accounting for 37.4% of the total share.
- Type Analysis: Based on type, In Vivo gene therapy led the segment with a 53.2% share in 2023, surpassing Ex Vivo approaches.
- End-Use Analysis: Among end-users, hospitals represented the largest share at 64.9% in 2023.
- Regional Analysis: North America is expected to capture approximately 38.30% of the market share during the forecast period.
Regional Analysis
North America is projected to hold approximately 38.30% of the market share during the forecast period. This growth can be attributed to the high prevalence of central nervous system (CNS) disorders in the region, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, which has created strong demand for innovative treatment options. The presence of a well-established healthcare infrastructure, significant research and development activities, substantial investment, and a favorable regulatory framework further support market expansion.
The United States, in particular, is experiencing a rising incidence of deaths associated with CNS disorders, which is expected to accelerate demand for effective therapies. The country accounts for the largest share of the North American market, followed by Canada.
In addition, the concentration of leading pharmaceutical companies actively investing in the development of advanced and targeted treatment solutions, coupled with high healthcare expenditure and a large patient base, are key factors driving the region’s growth.
Frequently Asked Questions on Gene Therapy in CNS Disorder
- What is gene therapy in CNS disorders?
Gene therapy in CNS disorders involves introducing, removing, or altering genetic material within a patient’s brain cells to correct faulty genes or regulate disease progression, offering long-term therapeutic effects beyond traditional symptom-based treatments. - Which CNS disorders can be treated with gene therapy?
Gene therapy shows potential in treating disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA), targeting genetic causes and offering disease-modifying benefits. - How does gene therapy work in the central nervous system?
Gene therapy uses viral vectors or genome editing tools to deliver functional genes directly to brain or spinal cord cells, aiming to repair defective genes, restore protein functions, and slow disease progression significantly. - What are the key benefits of gene therapy in CNS disorders?
Key benefits include targeting the root genetic causes of disease, potential for long-term symptom relief, reduced need for ongoing medications, and improved quality of life in patients suffering from chronic and progressive neurological conditions. - What is the growth rate of the gene therapy in CNS disorder market?
The market is expected to grow at a CAGR of 19.01% from 2024 to 2033, driven by rising prevalence of CNS disorders, advancements in gene therapy technologies, and increased investment in clinical development pipelines. - Which indication dominates the gene therapy in CNS disorder market?
Alzheimer’s disease is the leading indication, accounting for 37.4% of the total market share in 2023, due to the increasing patient population and urgent demand for disease-modifying therapies targeting genetic causes. - Which type of gene therapy holds the largest market share?
In Vivo gene therapy dominates the market with 53.2% share in 2023, owing to its efficiency in directly delivering genetic material to target cells, ensuring higher therapeutic impact and patient compliance compared to Ex Vivo approaches. - Which region is expected to lead the market during the forecast period?
North America is projected to capture approximately 38.3% of the global share, supported by advanced healthcare infrastructure, high prevalence of CNS disorders, strong research and development activity, and favorable regulatory support in the region.
Conclusion
Gene therapy in CNS disorders represents a transformative advancement in neurological medicine, addressing unmet needs where conventional treatments fall short. With the global market projected to grow from USD 8.4 billion in 2023 to USD 47.9 billion by 2033 at a CAGR of 19.01%, the sector demonstrates strong momentum.
Alzheimer’s disease leads indication share, while In Vivo therapies and hospital end-users dominate adoption. North America remains the leading region, driven by high disease prevalence, robust infrastructure, and active pharmaceutical investment. Despite cost and safety challenges, gene therapy is poised to reshape patient outcomes and redefine treatment paradigms worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



