Table of Contents
Overview
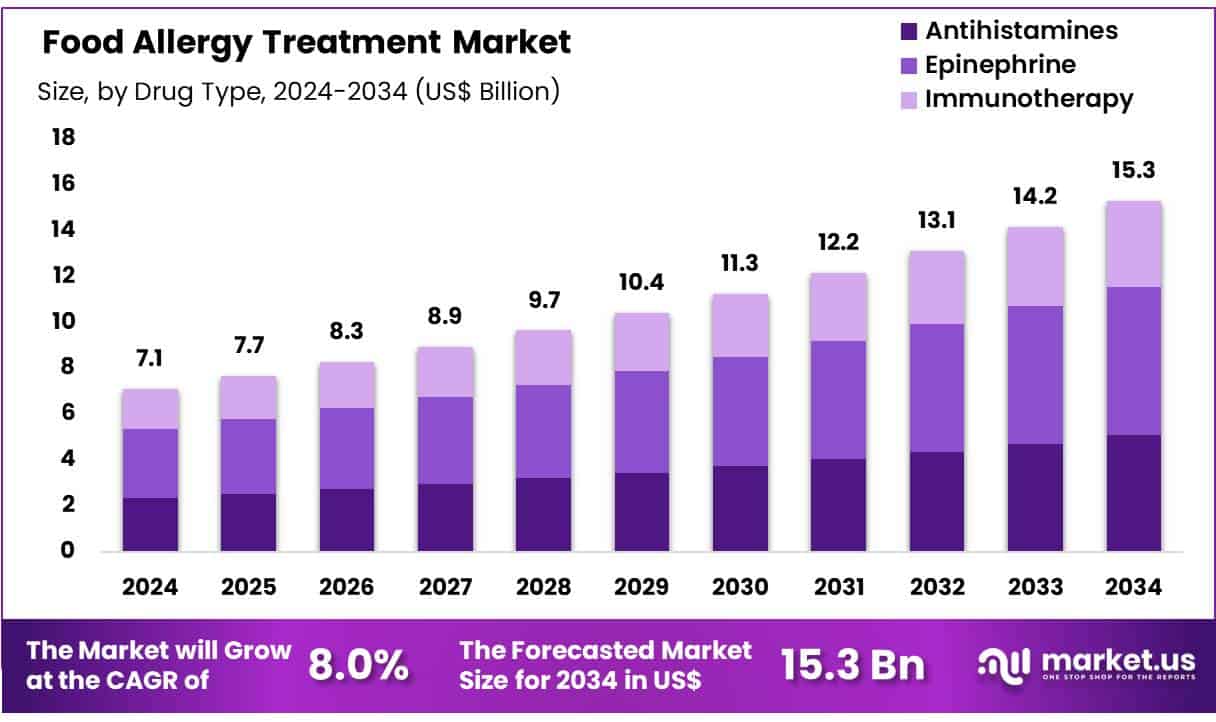
New York, NY – June 26, 2025 – Global Food Allergy Treatment Market size is expected to be worth around US$ 15.3 Billion by 2034 from US$ 7.1 Billion in 2024, growing at a CAGR of 8.0% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 40.5% share with a revenue of US$ 2.9 Billion.
The global healthcare community is witnessing growing momentum in the development of innovative food allergy treatments, addressing the urgent need for effective and long-lasting solutions for millions of patients worldwide. Food allergies affect approximately 250 million people globally, with symptoms ranging from mild reactions to life-threatening anaphylaxis. As prevalence continues to rise, particularly among children, the demand for novel therapies has intensified.
In response to this challenge, researchers and pharmaceutical companies are advancing a variety of treatment approaches, including oral immunotherapy (OIT), biologics such as anti-IgE monoclonal antibodies, and emerging peptide-based therapies. These treatments aim to desensitize patients to specific allergens, reduce allergic reactions, and ultimately improve quality of life.
In 2024, the U.S. FDA expanded approval for Palforzia, the first peanut allergy treatment, signaling a positive regulatory environment for food allergy therapeutics. Simultaneously, clinical trials for biologic agents targeting multiple food allergens are underway, demonstrating promising safety and efficacy profiles.
Global efforts are further supported by government-funded research, hospital-based allergy programs, and patient advocacy groups promoting awareness and early diagnosis. With increasing investment, supportive regulations, and advances in immunology, the food allergy treatment market is expected to witness sustained growth in the coming years. These developments represent a critical step toward safer and more inclusive food environments for allergic individuals.
Key Takeaways
- The global food allergy treatment market was valued at US$ 7.1 billion in 2024 and is projected to reach US$ 15.3 billion by 2034, expanding at a compound annual growth rate (CAGR) of 8.0% during the forecast period.
- Peanut allergy accounted for the largest share by allergen type, contributing approximately 21.2% of the total market revenue in 2024.
- By drug type, epinephrine emerged as the leading segment, representing about 42.4% of the global revenue share.
- In terms of the route of administration, the parenteral route dominated the market, accounting for nearly 53.0% of total sales in 2024.
- Among end users, hospital pharmacies captured the largest market share, holding around 47.8% of the global revenue.
- North America led the global market geographically, commanding over 40.5% of the total market revenue in 2024.
Segmentation Analysis
- Allergen Type Analysis: Peanut allergies accounted for 21.2% of the global food allergy treatment market in 2024, driven by their high prevalence and severity, especially in children. Treatments like oral immunotherapy and biologics such as Xolair® are showing promise in desensitization. FDA approvals like Palforzia® have expanded access to structured treatments. According to FARE, 6.1 million people in the U.S. have peanut allergies, reinforcing the demand for advanced therapies in this segment.
- Drug Type Analysis: In 2024, epinephrine held a 42.4% market share, making it the leading drug in food allergy management. Its fast-acting relief in severe reactions, especially anaphylaxis, makes it essential in emergency care. Epinephrine auto-injectors, such as EpiPen®, are widely adopted due to their effectiveness and ease of use. Recent innovations, like ARS Pharmaceuticals’ nasal spray Neffy, offer alternative delivery methods with comparable efficacy, enhancing patient options for emergency treatment.
- Route of Administration Analysis: The parenteral route dominated in 2024 with a 53.0% market share and is projected to grow at the fastest CAGR. This method ensures 100% bioavailability and rapid absorption, making it ideal for emergency allergy treatment, especially in anaphylaxis. Intramuscular injection of epinephrine via auto-injectors like EpiPen® is the standard approach due to its immediate action. The quick response time and high effectiveness continue to drive the dominance of parenteral delivery in severe allergy care.
- Distribution Channel Analysis: Hospital pharmacies led the market in 2024, accounting for 47.8% of total revenue. Their dominance stems from advanced infrastructure, high patient inflow, and specialized diagnostic and treatment capabilities. Hospitals manage severe allergy cases efficiently and support multidisciplinary care involving allergists and emergency teams. According to NCBI, 5–10% of inpatients had documented food allergies, often with multiple allergens. With government support and insurance coverage, hospitals remain key providers in food allergy treatment.
Market Segments
Allergen Type
- Dairy Products
- Poultry Products
- Tree Nuts
- Peanuts
- Shellfish & Fish
- Wheat
- Soy
- Others
Drug Type
- Antihistamines
- Epinephrine
- Immunotherapy
- Xolair
- PALFORZIA
- Others
Route of Administration
- Parenteral
- Oral
- Others
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Regional Analysis
In 2024, North America accounted for 40.5% of the global food allergy treatment market, maintaining the largest regional share. This dominance is attributed to advanced healthcare infrastructure, a high burden of food allergies, and rapid adoption of innovative therapies such as biologics and immunotherapies. The region also benefits from strong pharmaceutical R&D capabilities, well-established allergy clinics, and favorable reimbursement frameworks that support patient access to cutting-edge treatments.
Ongoing government initiatives, including FDA regulatory support and public awareness campaigns, have strengthened early diagnosis and treatment uptake. Skilled healthcare professionals and growing public education efforts further reinforce regional growth.
According to the CDC, nearly 5 million U.S. children—about one in thirteen—live with food allergies, highlighting the importance of continued advancements. Additionally, CDC data from 2021 reported that roughly 6% of both U.S. adults and children have food allergies, with Black, non-Hispanic individuals most commonly affected.
Emerging Trends
- Expansion of Oral Immunotherapy Approvals: The FDA has broadened indications for oral immunotherapy. Palforzia® (peanut allergen powder) was first approved in January 2020 for patients aged 4–17 years and, as of July 26, 2024, its use has been extended to children aged 1–3 years under a supplemental Biologics License Application. This regulatory progression underscores a trend toward earlier intervention in pediatric food allergy management.
- Proliferation of Clinical Research on Desensitization: A growing body of clinical trials is evaluating desensitization approaches. For example, a Phase 3, double-blind study (NCT03736447) is underway at 23 sites across North America and Europe to assess peanut oral immunotherapy. Simultaneously, patient registries (e.g., NCT03059589) are being used to gather real-world safety and efficacy data for food oral immunotherapy.
- Advancement of Epicutaneous Immunotherapy (EPIT): Epicutaneous patches delivering micro-doses of allergen through the skin are under investigation. A trial of VE416 (NCT03936998) is testing this method as either pretreatment or concurrent therapy to standard OIT, reflecting interest in less invasive delivery routes.
- Integration of Biologic Agents as Adjuncts: Monoclonal antibodies targeting IgE such as omalizumab have been approved to reduce the severity of food-allergic reactions. Their use alongside immunotherapy protocols is being explored to enhance safety and improve desensitization rates.
Use Cases
- Oral Immunotherapy with Palforzia®: In pivotal trials supporting approval, approximately 500 peanut-allergic participants received either Palforzia or placebo. After six months of maintenance dosing, 67.2 % of Palforzia recipients tolerated a 600 mg peanut-protein challenge without more than mild symptoms, compared with 4.0 % in the placebo group. This demonstrates the capacity of OIT to raise reaction thresholds and mitigate accidental exposures.
- Emergency Management with Epinephrine: Epinephrine remains the first-line treatment for acute food-allergy reactions. The CDC estimates that 1 in 13 U.S. children (about 8 %) has a food allergy, and at least 40 % of these children have required treatment in an emergency department for severe reactions. Consistent availability and prompt administration of epinephrine autoinjectors are therefore critical use cases in both clinical and community settings.
Conclusion
The global food allergy treatment market is experiencing robust growth, driven by rising prevalence, regulatory support, and advancements in therapeutic approaches such as oral immunotherapy, biologics, and parenteral drug delivery. With the market projected to reach US$ 15.3 billion by 2034, increasing investments, early intervention strategies, and patient awareness are shaping a more proactive and effective allergy management landscape.
North America remains the leading region, supported by strong healthcare infrastructure and research capabilities. Continued innovation, especially in pediatric care and biologic integration, is expected to enhance treatment outcomes and create safer environments for individuals living with food allergies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



