Table of Contents
Overview
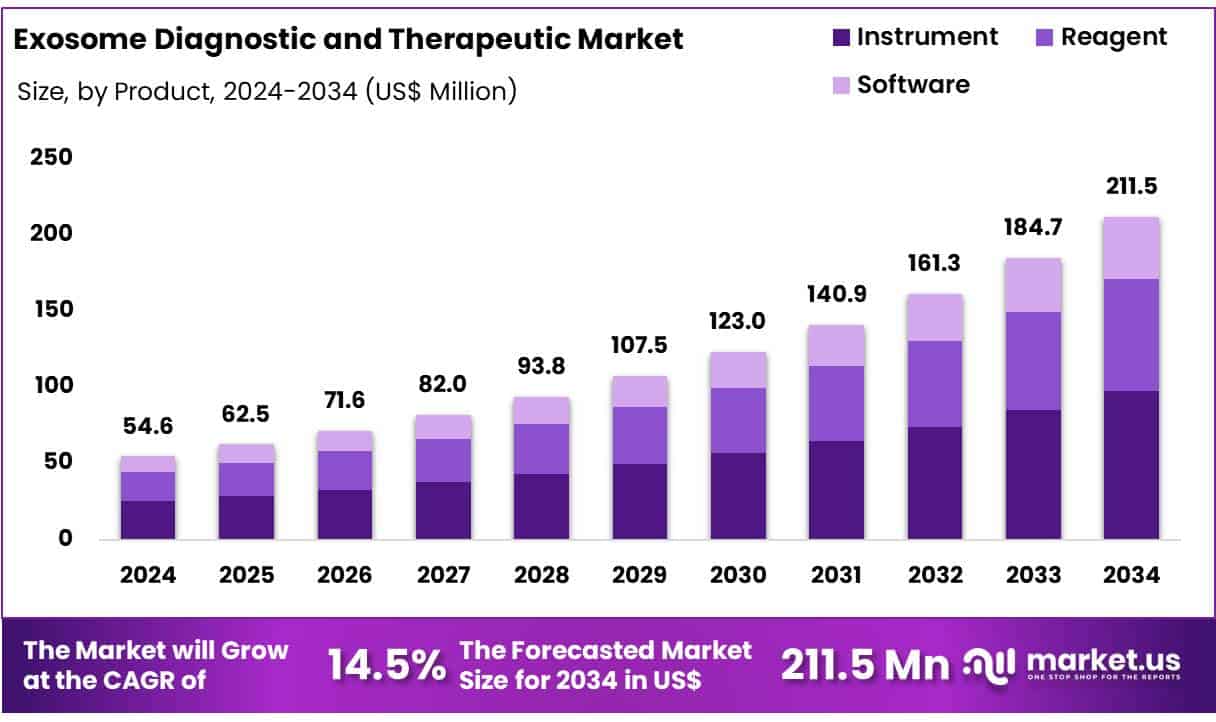
New York, NY – June 26, 2025 – Global Exosome Diagnostic and Therapeutic Market size is expected to be worth around US$ 211.5 Million by 2034 from US$ 54.6 Million in 2024, growing at a CAGR of 14.5% during the forecast period from 2025 to 2034. In 2024, North America led the market, achieving over 45.5% share with a revenue of US$ 440.0 Million.
The global exosome diagnostics and therapeutics market is experiencing rapid expansion, driven by growing demand for non-invasive diagnostic tools and targeted treatment approaches. Exosomes tiny extracellular vesicles released by cells have emerged as vital biomarkers in early disease detection, particularly in oncology, neurodegenerative, and cardiovascular conditions. Their role in intercellular communication and ability to carry genetic material, proteins, and lipids make them ideal candidates for liquid biopsy and drug delivery applications.
In 2024, the market is witnessing increased clinical interest, with pharmaceutical companies and academic institutions actively investing in exosome-based research. Exosome diagnostics are gaining traction for real-time monitoring of diseases such as prostate cancer and Alzheimer’s disease, while exosome therapeutics are showing promise in immunomodulation and regenerative medicine.
North America leads global adoption, supported by strong research funding, favorable regulatory policies, and advanced healthcare infrastructure. Meanwhile, Asia-Pacific is rapidly emerging due to growing biotech investments and expanding diagnostic laboratories.
As the field matures, strategic collaborations, advancements in isolation technologies, and supportive clinical trial data are accelerating product development and commercialization. The integration of exosomes into personalized medicine pipelines is expected to transform patient care and enable earlier, more accurate disease intervention.
Key Takeaways
- Market Size: The global exosome diagnostic and therapeutic market is projected to reach approximately US$ 211.5 million by 2034, rising from US$ 54.6 million in 2024.
- Market Growth: The market is expected to expand at a compound annual growth rate (CAGR) of 14.5% during the forecast period from 2025 to 2034.
- Product Analysis: In 2024, the instrument segment held the largest share of the exosome market, accounting for 45.4% of total revenue, driven by increasing use in exosome isolation and analysis.
- Application Analysis: The diagnostic application segment emerged as the leading area of use, representing 57.3% of total market applications in 2024, primarily due to rising demand for early disease detection and non-invasive testing.
- End-Use Analysis: Cancer institutes were the dominant end users in 2024, capturing 37.4% of overall market uptake, supported by the growing focus on tumor-derived exosome analysis in oncology research.
- Regional Analysis: North America led the global exosome diagnostic and therapeutic market in 2024, holding a 45.5% revenue share, equivalent to approximately US$ 440.0 million, driven by advanced research infrastructure and robust clinical adoption.
Segmentation Analysis
- Product Analysis: In 2024, instruments accounted for 45.4% of the exosome diagnostic and therapeutic market, driven by technologies such as ultracentrifuges, nanoparticle tracking analyzers, and microfluidic systems. These tools are regulated under FDA’s 21 CFR Part 820 to ensure consistent performance. Reagents held about 35%, comprising enrichment kits and RNA extraction solutions, enhancing assay reproducibility in NIH-funded studies. Software platforms enabled high-throughput analysis and AI-based biomarker discovery, forming a complete workflow for clinical and research applications.
- Application Analysis: Diagnostics dominated the application landscape with 57.3% share in 2024, largely due to non-invasive disease detection capabilities. Over 288 clinical trials registered on ClinicalTrials.gov focused on exosome-based diagnostics for cancer, cardiovascular, and neurological disorders. Many platforms demonstrated sensitivity above 90% using exosomal miRNA biomarkers. Therapeutic applications, accounting for 42.7%, centered on exosome-engineered drug delivery systems and regenerative therapies. Early-stage trials targeted conditions like myocardial infarction and autoimmune diseases, showing strong preclinical support for tissue repair and immune modulation.
- End-Use Analysis: Cancer institutes led the market in 2024 with a 37.4% share, notably among the 73 NCI-designated centers involved in advanced clinical research. Hospitals followed at 28.9%, with over 6,000 U.S. hospitals integrating exosome testing under CLIA-regulated diagnostic workflows. Diagnostic centers contributed 22.1%, supported by over 12,000 Medicare-certified labs performing routine biomarker assays. The remaining 11.6% comprised academic institutions, CROs, and biobanks, playing a vital role in preclinical studies and early therapeutic innovation using exosome platforms.
Market Segments
By Product
- Instrument
- Reagent
- Software
By Application
- Diagnostic
- Therapeutic
By End User
- Cancer Institute
- Hospital
- Diagnostic Center
- Others
Regional Analysis
In 2024, North America led the global exosome diagnostic and therapeutic market, holding over 45.5% market share, equivalent to US$ 440.0 million. This dominance is supported by strong federal research investment, including more than US$ 145 million from the NIH’s Extracellular RNA Communication program since 2013. The FDA’s regulatory guidance through the Center for Biologics Evaluation and Research has facilitated clinical translation, with hundreds of exosome-related trials listed on ClinicalTrials.gov, mostly led by U.S.-based institutions.
Europe followed with steady growth, bolstered by the EU’s Horizon Europe programme, which allocates €93.5 billion toward research and innovation from 2021 to 2027. A portion supports extracellular vesicle projects, aided by regulatory frameworks from the European Medicines Agency (EMA). National agencies in Germany, France, and the UK actively fund exosome-related diagnostics and regenerative therapies, supported by developed healthcare systems and advancing reimbursement mechanisms.
Asia-Pacific is an emerging high-growth region. China’s NMPA and regulatory frameworks now include exosome technologies. National R&D agencies across Japan, South Korea, and India have launched funding calls for clinical development. Expanded trial capacity and rising awareness of precision medicine are expected to drive robust market growth in the coming years.
Emerging Trends
Substantial Government Funding
The NIH Common Fund’s Extracellular RNA Communication Program has invested over US $145 million since 2013 in developing tools to isolate, analyze, and apply extracellular vesicles (including exosomes) for diagnostics and therapeutics. This funding has accelerated the creation of single-vesicle analysis technologies and standardized methods for biomarker discovery.
Expansion of Clinical Research
ClinicalTrials.gov now registers numerous exosome-related studies across multiple indications, reflecting diversified interest in both diagnostics and therapeutics. Key examples include:
- A urine test for prostate cancer risk (ExoDx™ Prostate IntelliScore, NCT03031418)
- Detection of EML4-ALK fusion exosomes in non-small cell lung cancer (NCT04499794)
- Focused ultrasound to enhance exosome delivery in depression (NCT04202770)
Strengthened Regulatory Oversight
The FDA has issued multiple public safety notifications and warning letters since 2019, clarifying that no exosome products are currently approved and emphasizing that any exosome-based intervention is regulated as a biological drug requiring pre-market approval. Clinics marketing unapproved exosome products have been subject to enforcement actions, underscoring a trend toward tighter regulatory scrutiny.
Use Cases
- Diagnostic Applications
Prostate Cancer Risk Assessment (NCT03031418): A urine-based exosome gene expression test is under evaluation to predict high-grade prostate cancer on initial biopsy. - Upper Gastrointestinal Tumor Detection (NCT06278064): Protein markers carried in exosomes are being profiled to enable early diagnosis and prognostication of esophageal and gastric cancers.
- Autoimmune Dry Eye Profiling (NCT06771427): Exosome proteomics is being studied to identify diagnostic signatures in Sjögren’s syndrome and dry eye disease.
Therapeutic Delivery and Treatment
- Targeted Cancer Therapy (NCT04499794): Exosomes are being engineered to carry oncogenic fusion proteins for precision treatment in NSCLC.
- Neuromodulation in Depression (NCT04202770): Focused ultrasound is used to guide exosome delivery to the brain in a pilot study for anxiety and depressive disorders.
- Pain Management in Neuralgia (NCT04202783): Intrathecal exosome injections are under investigation for craniofacial neuralgia, representing a novel non-opioid approach to chronic pain.
Conclusion
The global exosome diagnostics and therapeutics market is on a high-growth trajectory, projected to reach US$ 211.5 million by 2034. This growth is fueled by increasing demand for non-invasive diagnostics, advancements in exosome-based therapies, and robust research funding, especially in North America. Diagnostic applications dominate, supported by clinical trials and regulatory oversight.
Therapeutic use cases are rapidly expanding, with engineered exosomes enabling targeted drug delivery. As regulatory frameworks tighten and technologies mature, exosomes are poised to play a transformative role in personalized medicine, offering precise, early detection and innovative treatment strategies across a broad spectrum of diseases.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



