Table of Contents
Overview
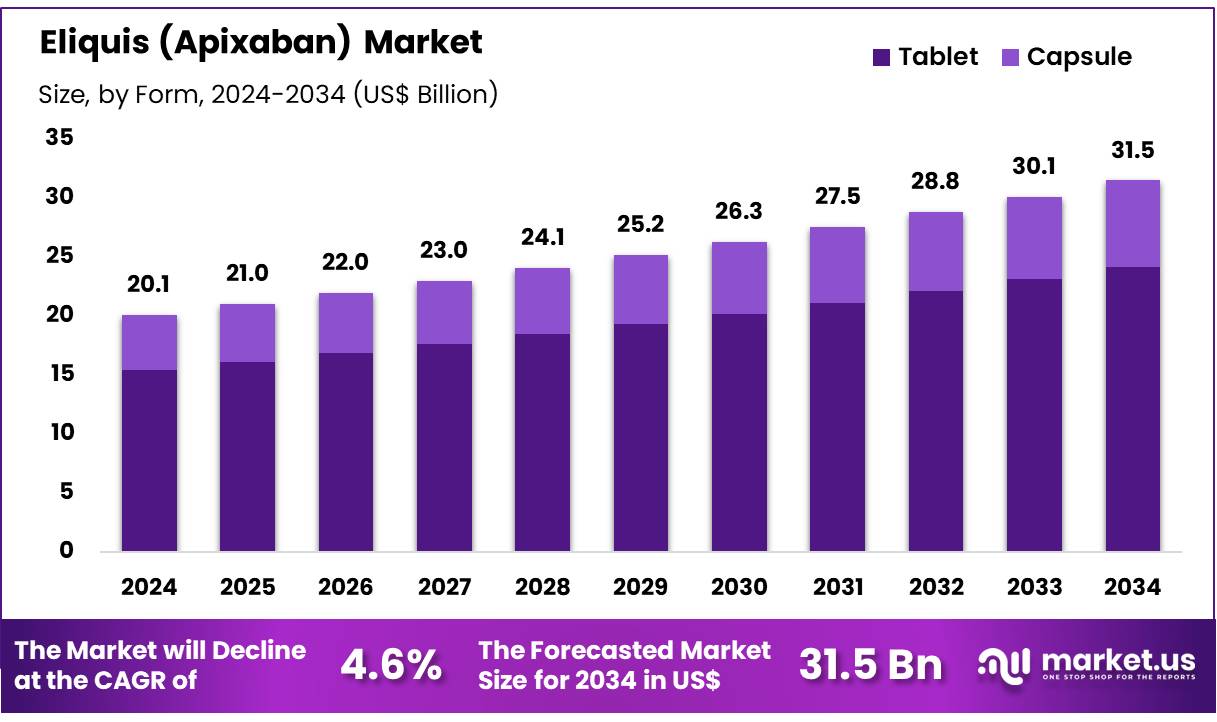
New York, NY – Dec 22, 2025 – Global Eliquis (Apixaban) Market size is forecasted to be valued at US$ 31.5 Billion by 2034 from US$ 20.1 Billion in 2024, growing at a CAGR of 4.6% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 34.1% share with a revenue of US$ 6.9 Billion.
Eliquis, generically known as Apixaban, is an oral anticoagulant widely prescribed for the prevention and treatment of thromboembolic disorders. The drug belongs to the class of direct Factor Xa inhibitors and is designed to reduce the risk of blood clot formation by selectively inhibiting Factor Xa, a key enzyme in the coagulation cascade.
Eliquis is primarily indicated for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, as well as for the treatment and secondary prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE). In addition, it is approved for thromboprophylaxis following hip or knee replacement surgeries. The efficacy and safety profile of the drug have been established through extensive clinical trials, demonstrating a reduced risk of stroke and major bleeding compared to traditional anticoagulant therapies in selected patient populations.
The product is jointly developed and commercialized by Bristol Myers Squibb and Pfizer. Since its introduction, Eliquis has achieved strong adoption across global markets, supported by favorable clinical outcomes, simplified dosing regimens, and the absence of routine coagulation monitoring requirements.
From a market perspective, Eliquis continues to hold a leading position within the novel oral anticoagulants (NOACs) segment. Market growth is being driven by the rising prevalence of cardiovascular disorders, an aging global population, and increasing physician preference for targeted oral anticoagulant therapies. As a result, Eliquis remains a key contributor to the evolving anticoagulation treatment landscape.
Key Takeaways
- In 2024, the Eliquis (Apixaban) market generated revenue of US$ 20.1 billion and is projected to grow at a declining CAGR of 4.6%, reaching US$ 31.5 billion by 2034.
- Among indications, Atrial Fibrillation (AF) emerged as the leading segment, accounting for 58.9% of the total market share in 2024.
- Tablets represented the dominant dosage form, holding a 76.7% market share in 2024.
- By route of administration, the oral segment led the market with a share of 87.1% in 2024.
- Based on distribution channel, retail pharmacies constituted the largest segment, capturing 54.8% of the market in 2024.
- North America dominated the global market, holding the highest regional share of 34.1% in 2024.
Regional Analysis
North America Leading the Eliquis (Apixaban) Market
North America, led primarily by the United States, continues to hold a dominant position in the apixaban market. This leadership is largely driven by the high prevalence of cardiovascular conditions, including atrial fibrillation, deep vein thrombosis, and pulmonary embolism. According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects up to 900,000 individuals annually in the United States, with an estimated 60,000 to 100,000 associated deaths each year. A significant proportion of patients also experience long-term complications, further increasing the clinical burden.
The rising incidence of these conditions is expected to continue, supported by an aging population, thereby sustaining demand for effective anticoagulant therapies. The region benefits from a well-developed healthcare infrastructure, which supports the widespread adoption of innovative treatments such as apixaban. In addition, strong pharmaceutical distribution networks and broad insurance coverage enhance patient access and treatment adherence, reinforcing market growth in North America.
Asia Pacific Expected to Register the Highest CAGR
The Asia Pacific (APAC) region is projected to witness the fastest growth in the apixaban market during the forecast period. This expansion is driven by a growing burden of cardiovascular diseases, particularly atrial fibrillation, across countries such as China, India, Japan, and South Korea. Demographic shifts, including rapidly aging populations, along with urbanization and lifestyle changes such as sedentary behavior and dietary imbalances, are contributing to the rising incidence of these conditions.
Consequently, demand for effective anticoagulation therapies is increasing across the region. Although the adoption of direct oral anticoagulants (DOACs) such as apixaban was initially slower in APAC compared to Western markets, awareness among healthcare professionals and patients is improving, leading to accelerated uptake.
The availability of generic apixaban formulations in markets such as India has further supported accessibility. In addition, regulatory developments have strengthened the global supply landscape. In March 2023, Zydus Lifesciences announced that it had received approval from the U.S. Food and Drug Administration to market a generic version of apixaban for the prevention and treatment of blood clots. The approved tablets, offered in 2.5 mg and 5 mg strengths, are manufactured at the company’s formulation facility in Moraiya, Ahmedabad, India.
Emerging Trends
- Expansion into Pediatric Venous Thromboembolism (VTE): In April 2025, regulatory approval in the United States was extended to include pediatric VTE treatment and recurrence prevention from birth onward, following a minimum of five days of initial anticoagulation. This development significantly broadens clinical use into younger patient populations and addresses an unmet need in pediatric anticoagulation.
- Introduction of Direct-to-Patient Access Program: Beginning September 8, 2025, eligible patients in the United States will be able to purchase the therapy directly through the “Eliquis 360 Support” program. The initiative offers pricing more than 40% below the list price, combined with nationwide delivery, thereby enhancing affordability and improving patient access.
- Growth in Prescriptions and Market Uptake: Global utilization continues to rise, supported by a favorable safety profile, predictable pharmacokinetics, and simplified dosing compared with vitamin K antagonists. These attributes have contributed to improved patient adherence and consistently positive clinical outcomes across major healthcare markets.
- Evolving Cost Dynamics Influenced by Generics and Policy Measures: Pricing pressure is increasing due to the entry of generic apixaban products and policy-driven reforms. Notable developments include its position as the most prescribed oral anticoagulant in Australia and a negotiated U.S. Medicare price reduction of approximately 56% for 2026 relative to 2023 levels.
- Real-World Evidence from Comparative Clinical Studies: A collaborative study between the U.S. Department of Veterans Affairs and the FDA, expected to launch in spring 2025, will evaluate apixaban versus rivaroxaban in patients with nonvalvular atrial fibrillation. The study is designed to generate real-world evidence on comparative safety, effectiveness, and long-term outcomes to inform clinical decision-making.
Use Cases
- Prevention of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation (NVAF): The therapy is indicated for reducing the risk of stroke and systemic embolism in adults with NVAF and is widely adopted as an alternative to warfarin due to a lower incidence of major bleeding and a more consistent anticoagulant effect.
- Postoperative Prophylaxis of Deep Vein Thrombosis (DVT): It is approved for the prevention of DVT and associated pulmonary embolism in adults undergoing hip or knee replacement surgery, supporting improved postoperative recovery by minimizing clot-related complications.
- Treatment and Secondary Prevention of Venous Thromboembolism (VTE): The agent is used for the treatment of deep vein thrombosis and pulmonary embolism in adults and for reducing the risk of recurrence following completion of initial therapy, enabling both acute management and long-term risk reduction.
- Pediatric VTE Treatment and Recurrence Prevention: Recent approval for pediatric use allows treatment and prevention of recurrent VTE from birth onward after initial anticoagulation, addressing a critical therapeutic gap in pediatric care.
- Emergency and Surgical Management via Hemoadsorption: In urgent cardiac surgical settings, hemoadsorption techniques such as Cytosorb have been utilized to reduce circulating drug levels, thereby lowering bleeding risk and facilitating safer surgical intervention when rapid anticoagulant reduction is required.
Frequently Asked Questions on Eliquis (Apixaban)
- How does Eliquis (Apixaban) work in the body?
Eliquis functions by selectively inhibiting factor Xa, a key enzyme in the coagulation cascade. This mechanism prevents thrombin generation and clot formation, enabling effective anticoagulation with a predictable pharmacological profile and reduced monitoring requirements. - What are the key clinical benefits of Eliquis compared to warfarin?
Clinical studies indicate that Eliquis demonstrates lower rates of major bleeding and intracranial hemorrhage compared to warfarin, while maintaining comparable or superior efficacy in stroke prevention, thereby improving patient safety and long-term treatment adherence. - What are the common side effects associated with Eliquis?
The most frequently reported adverse effects include bleeding-related events such as bruising, epistaxis, and gastrointestinal bleeding. The overall safety profile is considered favorable when dosing guidelines and contraindications are strictly followed in clinical practice. - What factors are driving growth in the Eliquis (Apixaban) market?
Market growth is driven by the rising prevalence of atrial fibrillation, increasing incidence of venous thromboembolism, aging populations, and strong physician preference for direct oral anticoagulants offering improved safety and convenience over traditional therapies. - How does Eliquis perform competitively in the global anticoagulant market?
Eliquis holds a leading market share within the direct oral anticoagulant segment, supported by robust clinical evidence, broad regulatory approvals, and strong commercialization efforts by major pharmaceutical companies across developed and emerging healthcare markets. - What impact will patent expiration have on the Eliquis market?
Patent expiration is expected to intensify price competition due to generic entry, leading to revenue pressure. However, sustained demand is anticipated due to established prescribing patterns, brand recognition, and continued clinical reliance in high-risk patient populations. - Which regions contribute most significantly to Eliquis market revenues?
North America and Europe represent the largest revenue contributors, supported by high diagnosis rates and reimbursement coverage. Meanwhile, Asia-Pacific markets are projected to witness faster growth due to improving healthcare access and expanding cardiovascular disease awareness.
Conclusion
Eliquis (apixaban) has established itself as a cornerstone therapy in the global anticoagulation landscape, supported by strong clinical evidence, broad regulatory approvals, and widespread physician acceptance. Sustained market leadership is underpinned by its proven efficacy in atrial fibrillation and venous thromboembolism, favorable safety profile, and ease of oral administration.
While patent expiry and generic competition are expected to exert pricing pressure, expanding indications, including pediatric use, and improved patient access initiatives will continue to support demand. Overall, Eliquis is expected to remain a clinically and commercially significant anticoagulant over the long term.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



