Table of Contents
Introduction
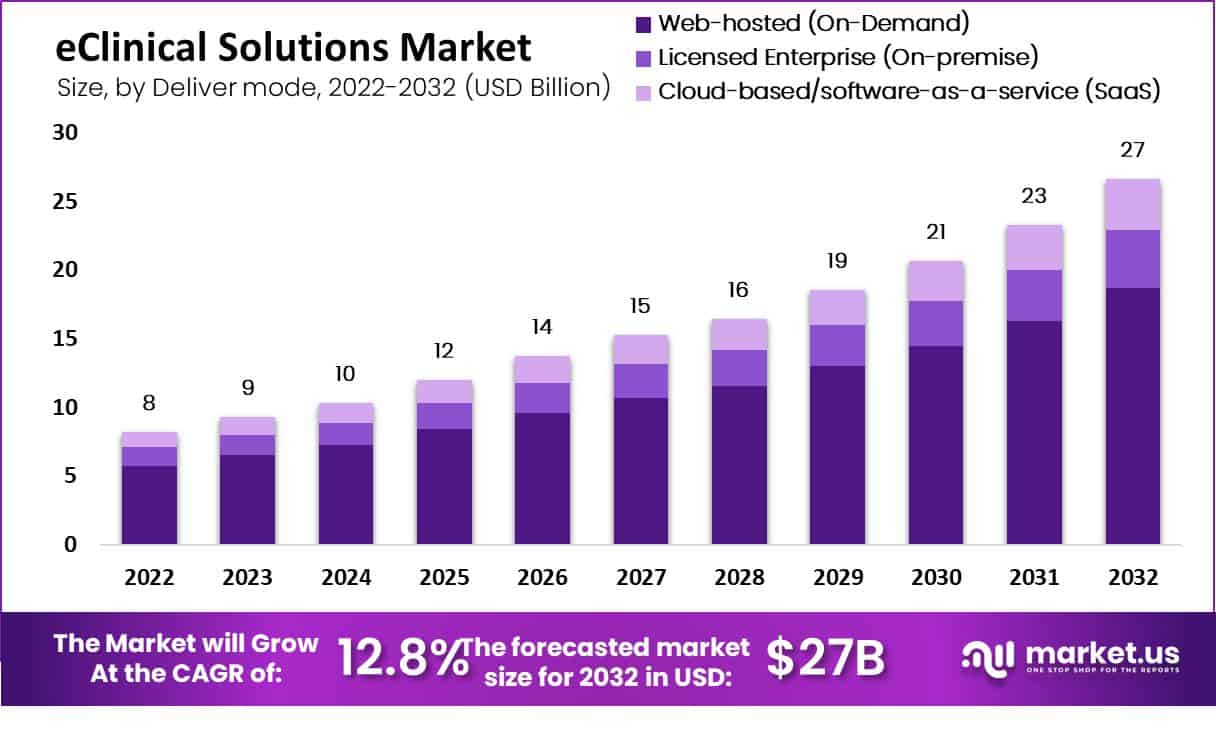
The global eClinical Solutions Market is poised for substantial growth, with its valuation projected to reach approximately USD 27 billion by 2032, up from USD 8 billion in 2022. This represents a compound annual growth rate of 12.8% over the decade. This surge is primarily fueled by the increasing number of clinical trials worldwide, necessitating efficient trial management systems. The World Health Organization reports over 380,000 registered clinical trials, highlighting the escalating demand for robust eClinical solutions that enhance data management and trial efficiency.
Government initiatives are also pivotal in driving the adoption of eClinical solutions. For instance, the U.S. FDA’s regulations on electronic records and signatures have catalyzed the integration of digital platforms in clinical settings. Additionally, the integration of advanced technologies such as artificial intelligence (AI) and machine learning (ML) into eClinical platforms is revolutionizing trial processes. These technologies improve data accuracy and operational efficiency, further supported by the U.S. Department of Health and Human Services’ push for digital transformation in healthcare.
The market’s expansion is significantly aided by pharmaceutical giants investing in cloud-based eClinical solutions to expedite processes, as seen with Pfizer and Moderna during the COVID-19 vaccine trials. These instances demonstrate the platforms’ capability to accelerate research and development timelines. Furthermore, the trend of digitalization in healthcare is anticipated to continue, underpinned by strategic partnerships aimed at fostering technological advancements. Collaborations like that between Microsoft and Novartis are instrumental in developing AI-powered tools for clinical trials.
In 2024, the market witnessed strategic developments that emphasize the shift towards digitalization and patient-centered research. Medidata Solutions, part of Dassault Systèmes, launched a new platform enhancing patient engagement across various life sciences phases, from R&D to commercialization. This reflects a broader industry move towards more integrated, patient-focused research models.
Other notable developments include Danaher’s acquisition of Abcam plc for $5.7 billion, expected to close by mid-2024. This acquisition will expand Danaher’s life sciences portfolio, integrating Abcam’s extensive biotechnology products. Additionally, Signant Health acquired DSG in July 2023, gaining advanced electronic data capture (EDC) and direct data capture (DDC) technologies. This enhances Signant’s ability to offer comprehensive digital solutions, optimizing data integrity and trial efficacy in both traditional and decentralized trial settings. These actions underscore a robust industry trend towards enhancing clinical trial efficiency through innovative eClinical solutions.
Key Takeaways
- By 2032, the eClinical Solutions Market is projected to grow to USD 27 billion from USD 8 billion in 2022, with a CAGR of 12.8%.
- Electronic Clinical Outcome Assessment (eCoA) leads in the eClinical market, focusing on enhancing the quality of clinical data collection.
- In 2022, web-hosted (on-demand) solutions were preferred for their flexibility and cost savings; cloud-based solutions anticipate the highest growth rate at 13.4%.
- Phase III clinical trials were predominant in 2022, while Phase I trials are expected to grow at the fastest rate of 13.0%.
- Contract Research Organizations (CROs) held a 36.7% market share in 2022 and are predicted to expand rapidly at a 15.1% CAGR.
- North America led the market in 2022 with a 52% revenue share, while the Asia-Pacific region is expected to surge at a 15.4% CAGR.
eClinical Solutions Statistics
- The market size in 2022 was valued at $8 billion.
- The market size in 2023 is estimated to reach $9 billion.
- The market size in 2024 is projected to be $10 billion.
- The market size in 2025 is forecasted to be $12 billion.
- The market size in 2026 is expected to reach $14 billion.
- The market size in 2027 is projected to grow to $15 billion.
- The market size in 2028 is expected to be $16 billion.
- The market size in 2029 is forecasted to be $19 billion.
- The market size in 2030 is projected to reach $21 billion.
- The market size in 2031 is expected to be $23 billion.
- The market size in 2032 is forecasted to be $27 billion.
- The market is expected to grow at a 12.8% CAGR from 2022 to 2032.
Emerging Trends
- Automation and Efficiency in eClinical Solutions: The eClinical solutions sector is increasingly embracing automation, particularly focusing on database builds, technical documentation, and operational reporting. This trend aims to enhance overall efficiency by significantly reducing the time and resources dedicated to various clinical trial processes. Automation not only streamlines workflows but also minimizes human error, ensuring higher accuracy and reliability in trial outcomes. As the industry progresses, this move towards automated systems is expected to become a standard practice, reshaping how clinical trials are conducted.
- Advancements in Data Management Tools: In response to the growing complexity and volume of data in clinical trials, there is a notable shift towards more advanced data management tools. These tools are designed to efficiently handle diverse data sources, providing a robust framework for managing vast amounts of information. The integration of sophisticated data management solutions is crucial for achieving timely and accurate trial outcomes, enabling researchers to make informed decisions quickly and effectively. This trend highlights the ongoing evolution of data handling practices within the eClinical solutions landscape.
- Rise of Decentralized Clinical Trials: Decentralized clinical trials (DCT) are becoming increasingly popular, driven by a patient-centered approach and supported by technological advancements that facilitate remote participation and data collection. This model offers greater convenience for participants and can lead to higher engagement rates. The shift towards DCT is a response to the need for more flexible and accessible trial formats, which are essential for accommodating diverse participant needs and improving trial efficiency.
- AI and Digital Twins in Clinical Trials: Artificial intelligence (AI), especially through the use of digital twins, is poised to transform clinical trials. These technologies enable precise simulations and predictions, which can reduce the time, cost, and complexity of trials. Digital twins create virtual representations of clinical trial processes, allowing for detailed analysis and scenario testing without the risks associated with traditional methods. This innovative approach is expected to enhance trial design and execution, making them more effective and efficient.
- Enhancing Data Security and Privacy: As clinical trials become more data-intensive, the importance of data security and privacy is escalating. The industry is implementing stronger measures to ensure the protection of patient data, complying with increasingly stringent regulatory requirements. Enhancing data security involves sophisticated encryption methods, secure data storage solutions, and comprehensive data governance frameworks. This trend is critical for maintaining trust and integrity within clinical trials.
- Focus on Talent Development in Data Management: The demand for skilled professionals in data management and analysis is growing rapidly within the eClinical solutions field. Companies are investing in training and development programs to address this talent gap. By enhancing the skills of their workforce, organizations can better manage the complexities of modern clinical trials, ensuring they have the expertise needed to handle advanced data systems and analytics tools. This focus on talent development is essential for sustaining innovation and maintaining a competitive edge in the industry.
Use Cases
- Streamlined Clinical Trial Management: eClinical solutions revolutionize the management of clinical trials by introducing automated systems that streamline the setup, data collection, and monitoring processes. These technological advancements allow for quicker trial initiation and more efficient management, significantly reducing the time and manpower typically required. The integration of eClinical platforms ensures that all trial aspects-from patient enrollment to data analysis-are managed effectively, minimizing errors and enhancing the overall trial workflow. This results in faster completion times and potentially earlier drug development stages, leading to quicker patient access to innovative treatments.
- Enhanced Data Analysis: Advanced analytics integrated into eClinical solutions empower researchers to handle and interpret large volumes of data swiftly. By utilizing these powerful tools, trial teams can derive actionable insights from complex datasets, facilitating a quicker decision-making process. This capability is crucial for identifying trends, predicting outcomes, and making informed adjustments during trials, which ultimately improves the quality and efficacy of the results. Enhanced data analysis provided by eClinical solutions ensures that clinical trials can adapt to new information rapidly, optimizing research strategies and outcomes.
- Remote Patient Monitoring: The use of eClinical solutions extends beyond traditional clinical environments into patients’ daily lives through mobile health applications and wearable technologies. These tools enable continuous monitoring of patient health data in real-time, significantly increasing the accuracy and reliability of the data collected. Remote patient monitoring not only engages participants more actively in their health management but also allows for the collection of a broader range of data points across diverse environments. This approach enhances the understanding of treatment effects in real-world settings, improving the generalizability and applicability of trial findings.
- Regulatory Compliance: eClinical solutions play a pivotal role in maintaining strict adherence to ever-evolving global regulatory standards. These platforms offer robust features that ensure data integrity and security, essential for compliance with regulatory bodies. Automated systems facilitate real-time data tracking and reporting, which is crucial for audit readiness and regulatory reviews. By integrating these solutions, organizations can safeguard against compliance risks, avoid potential fines, and maintain a trustworthy reputation, all while accelerating the pace of clinical research by ensuring that regulatory hurdles do not cause unnecessary delays.
- Personalized Medicine: Personalized medicine, tailored to individual patient needs, is at the forefront of modern healthcare innovations, supported extensively by eClinical solutions. These platforms utilize vast datasets and artificial intelligence to develop customized treatment plans that significantly enhance treatment efficacy and patient outcomes. By analyzing individual patient data, eClinical solutions identify unique biomarkers and health patterns that predict the best therapeutic approaches, making treatments not only more effective but also reducing the likelihood of adverse effects. This personalized approach underscores the transformative potential of eClinical solutions in advancing healthcare towards more targeted and effective interventions.
Conclusion
In conclusion, the eClinical Solutions Market is set for significant expansion, driven by the growing necessity for advanced clinical trial management systems amid an increasing number of worldwide clinical trials. The integration of technologies such as artificial intelligence and cloud computing is transforming the landscape, improving data management, and trial efficiencies. The market’s growth is further supported by strong government backing and strategic industry collaborations, aiming to enhance digitalization and patient-centric research approaches. As the sector continues to evolve, the adoption of eClinical solutions is expected to rise, marking a pivotal shift towards more efficient and effective clinical research methodologies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



