Table of Contents
Overview
New York, NY – Aug 04, 2025: The global Early Toxicity Testing Market is projected to reach US$ 2.7 Billion by 2034, up from US$ 1.4 billion in 2024. This growth reflects a CAGR of 6.8% during the forecast period from 2025 to 2034. North America dominates the market with over 39.2% share, holding a value of US$ 0.5 billion. Growth is driven by rising drug development costs and the need to detect toxic compounds early. Early-stage screening reduces downstream failures and improves regulatory approval chances.
Early toxicity testing is vital for investigational new drug (IND) applications and chemical safety assessments. It ensures drugs meet safety standards set by global regulators like the FDA, EMA, and OECD. These evaluations are conducted before human clinical trials begin. They help detect risks early, enhance patient safety, and reduce overall development costs. Regulatory authorities require solid preclinical data to justify human testing. This step is essential for market entry and ensures compliance with global safety protocols.
According to FDA guidelines, IND filings must include detailed pharmacological and toxicological data. Most of these data are collected from animal models during preclinical studies. These studies aim to show that a drug is reasonably safe for first-in-human trials. They must prove that the potential benefits outweigh the risks. This ensures that only the most promising compounds enter clinical testing. It also helps pharmaceutical companies avoid costly failures and improves the overall efficiency of drug development pipelines.
Innovative methods are transforming how toxicity is assessed. In vitro techniques like MTT, MTS, and ATP assays are now widely used. These tests evaluate cell viability and metabolic activity without using animals. Neutral red uptake checks membrane integrity, while ELISA-based cytokine assays detect inflammation. These methods offer reproducible results and are more ethical alternatives to animal testing. They allow for faster, early-stage screening, making drug discovery more efficient and cost-effective. In vitro testing is becoming a foundation for modern toxicology.
In silico toxicology is also growing fast. Computational tools use chemical structure data to predict toxicity. Programs like the EPA’s ToxCast screened over 1,000 chemicals with up to 82% accuracy. Europe’s eTOX project created similar models using curated toxicology data. Organ-on-a-chip (OoC) is another breakthrough. Devices like liver-on-a-chip simulate human organ responses more accurately than animal models. These platforms are now gaining FDA recognition. They reduce animal use and improve safety data, boosting both ethics and accuracy in drug development.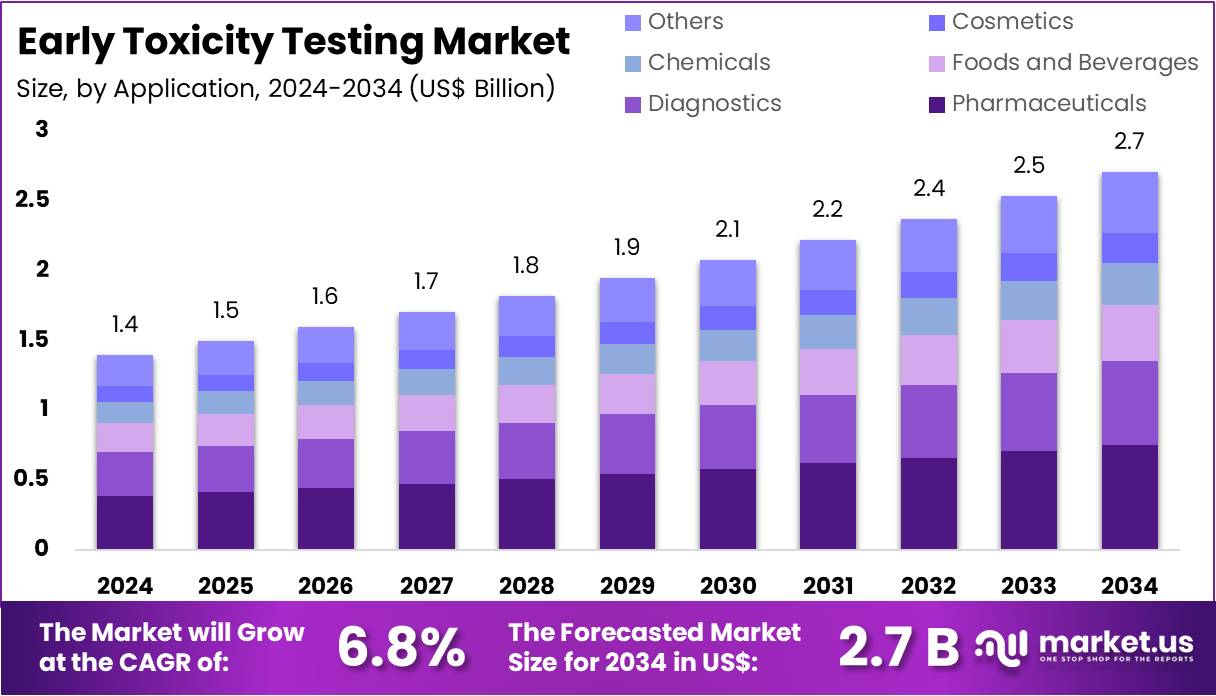
Key Takeaways
- Industry analysts expect the global Early Toxicity Testing market to reach US$ 2.7 billion by 2034, rising from US$ 1.4 billion in 2024.
- The market is set to grow at a steady CAGR of 6.8% between 2025 and 2034, reflecting rising demand for safer drug development.
- In-vitro testing methods took the lead in 2024, capturing over 38.2% of the total market share within the Type segment.
- The Pharmaceuticals sector held the largest application share in 2024, accounting for more than 28.4% of the Early Toxicity Testing market.
- North America dominated regional revenues in 2024, with a 39.2% market share valued at approximately US$ 0.5 billion.
Regional Analysis
North America led the global Early Toxicity Testing market, accounting for over 39.2% share and holding a market value of US$ 0.5 billion. This dominance is largely due to the region’s robust pharmaceutical and biotechnology industries, along with a well-established preclinical research infrastructure. Regulatory agencies like the U.S. FDA provide clear guidelines that promote the adoption of early toxicity testing. These frameworks support the integration of advanced, ethical testing methods that help accelerate drug development and ensure higher safety standards.
The United States continues to be the primary growth driver within the region, backed by high R&D spending and strong institutional focus on in-vitro and cell-based models. These approaches enhance predictive accuracy while reducing reliance on animal testing. In parallel, Canada supports the regional market through research funding, automation initiatives, and regulatory alignment with global standards. These efforts have attracted both domestic and international pharmaceutical firms. Together, these developments are expected to reinforce North America’s leadership in early toxicity testing in the coming years.
Segmentation Analysis
The In-vitro segment dominated the Type segment of the Early Toxicity Testing Market with a market share exceeding 38.2%. This dominance is driven by the increasing use of lab-based cell and tissue models, which offer cost-effective, faster, and ethically aligned alternatives to animal testing. Regulatory support from agencies like the FDA has further encouraged adoption by emphasizing accuracy and reproducibility in early drug development. Meanwhile, the In-vivo segment maintained a strong presence due to its continued role in assessing systemic and organ-specific toxicity. Although animal use remains controversial, it is still essential in later preclinical phases. The In-silico segment is also gaining momentum, with AI-driven models offering predictive insights based on chemical and biological data. While not yet fully regulatory-approved, these tools enhance speed and scalability in early-stage toxicology screening.
From an application perspective, the Pharmaceuticals segment led the market in 2024, capturing over 28.4% share. This leadership stems from the increasing demand for detailed preclinical safety evaluations, helping companies minimize late-stage failures. Diagnostics also showed significant growth, fueled by the rise of molecular testing and personalized medicine, which demand early validation of safety. Outside healthcare, industries like cosmetics, chemicals, and food and beverages are turning to alternative testing methods due to strict animal testing bans, especially in the EU. These sectors now rely on in-vitro and tissue-engineered assays to meet regulatory standards and ensure public safety.
Key Players Analysis
The Early Toxicity Testing market is driven by several key players, with Thermo Fisher Scientific taking a leading role through its comprehensive solutions in predictive toxicology. The company offers advanced platforms such as high-content screening, assay development, and integrated analytical workflows that enhance testing accuracy and accelerate drug discovery. Its wide portfolio of reagents and services supports pharmaceutical research at multiple levels. Continuous investment in automation and innovation further strengthens Thermo Fisher’s leadership in early-stage safety profiling. BD Biosciences is another major contributor, specializing in flow cytometry and cell-based assays. Its technologies enable precise cellular response analysis, including immune profiling and apoptosis detection. With a focus on analytical accuracy and single-cell insights, BD plays a vital role in advancing preclinical toxicology research.
Covance, a Labcorp division, is a prominent contract research organization offering both in vitro and in vivo testing, pharmacokinetic analysis, and biomarker identification. Its global, GLP-compliant facilities support early-phase drug development and regulatory submissions. Covance’s strong collaborations with pharmaceutical companies enhance pipeline efficiency and ensure regulatory alignment. Additionally, Agilent Technologies and Bio-Rad Laboratories contribute through advanced analytical tools. Agilent provides high-precision chromatography and mass spectrometry systems, while Bio-Rad offers PCR platforms and ELISA kits for toxicology screening. Together, these players address industry needs for scalable, ethical, and accurate early-stage testing solutions.
Emerging Trends
- Shift Toward In-Vitro Testing: Many companies are now moving away from animal testing and choosing in-vitro methods. These involve using lab-grown human cells and tissues to study how chemicals affect the body. In-vitro testing is faster, more affordable, and more ethical than traditional animal studies. It also provides more accurate data in early drug development. Regulatory agencies are encouraging its use because it improves safety predictions. Currently, in-vitro methods are used in over 35% of early-stage toxicity screening. This trend is expected to grow as more pharmaceutical companies focus on cost-effective and humane solutions during the preclinical phase.
- Adoption of Organ-on-a-Chip (OoC): Organ-on-a-chip (OoC) devices are transforming early toxicity testing. These tiny chips simulate human organs like the liver, heart, or lungs. They use microfluidics to mimic blood flow and real-time organ behavior. OoC models give more accurate results than animal tests, especially for complex human responses. Drug developers use them to test toxicity earlier and avoid late-stage failures. These chips also reduce the need for animal experiments. As a result, they are gaining attention across biotech and pharmaceutical research. More companies are adopting OoC technology to improve data quality and shorten development timelines.
- In-Silico Modeling Is Gaining Ground: In-silico modeling is becoming a popular tool in toxicology research. These computer-based methods use AI and chemical structure data to predict how toxic a substance might be. In-silico tools are fast, scalable, and low-cost. They help scientists screen many compounds quickly without using live tests. Some models can reduce wet-lab experiments by up to 40% in early R&D. While not fully accepted by regulators yet, these tools support early-stage decision-making. As artificial intelligence improves, in-silico methods will likely play a larger role in drug safety screening and chemical risk assessment.
- Regulatory Acceptance of Alternative Methods: Regulatory agencies are starting to accept non-animal testing methods for early toxicity studies. Organizations like the FDA and EMA are updating their guidelines to include new technologies. Tools that simulate organ damage, like drug-induced liver injury, are being considered for formal review. This shift encourages companies to invest in ethical and modern testing models. Regulatory support also helps reduce the time and cost of drug development. As policies become more flexible, alternative methods such as in-vitro, in-silico, and organ-on-a-chip systems are gaining widespread validation in the toxicology space.
- Focus on Personalized Toxicity Profiling: Personalized toxicity testing is emerging as a powerful trend in drug development. Companies are using patient-specific cell models to study how individuals may react to a drug. These customized tests help predict side effects before clinical trials begin. They also reduce the risk of unexpected adverse reactions in human subjects. As personalized medicine becomes more common, this testing approach is growing in demand. It supports safer, targeted therapies for smaller patient groups. Early toxicity insights based on real patient biology improve both drug safety and success rates in development pipelines.
Use Cases
- Pharmaceutical Drug Development: Early toxicity testing is a critical step before human clinical trials can begin. All drug candidates must pass safety assessments in the preclinical stage. About 70% of compounds fail during this phase, mainly due to toxicity issues. By identifying harmful substances early, companies can save time and resources. In-vitro and in-silico methods are often used to detect toxicity before moving to animal or human trials. These tools help reduce failures in later stages and improve the chance of regulatory approval. Early testing ensures only the safest and most promising drugs advance in development.
- Cosmetic Product Safety: The cosmetic industry relies heavily on early toxicity testing due to strict bans on animal testing, especially in the European Union. Companies now use in-vitro methods to check for skin and eye irritation. These tests use human cell models to provide accurate safety data. Over 90% of new cosmetic products in regulated markets are screened using these techniques. Early testing allows for faster product launches and better compliance with international safety rules. It also helps brands maintain ethical standards while protecting consumer health from potential irritants or allergic reactions.
- Food Additive and Chemical Safety: Food and beverage companies must ensure that ingredients like preservatives, colorants, and additives are safe for consumption. Early toxicity testing plays a vital role in this process. Cell-based assays can detect harmful effects at very low concentrations sometimes as low as 1–10 µg/mL. These tests help prevent dangerous compounds from reaching the market. They also support regulatory compliance and quality assurance. Using advanced screening methods, manufacturers can evaluate ingredient safety early in the product cycle. This protects consumers and builds trust in food safety standards globally.
- Environmental Chemical Assessment: Toxicity testing is essential for evaluating pesticides, industrial chemicals, and pollutants. These substances can affect both humans and ecosystems. Early screening helps identify potential risks before full-scale production or release. It also supports faster regulatory approvals by reducing testing time by 25–30% in many workflows. In-vitro and in-silico tools allow scientists to assess environmental impact efficiently. These methods are especially useful for studying water, soil, and air contamination. With early testing, harmful chemicals can be modified or eliminated, ensuring better protection for public health and the environment.
FAQs About Early Toxicity Testing
1. What is early toxicity testing?
Ans:- Early toxicity testing refers to the assessment of a substance’s potential to cause harmful effects before it proceeds to human clinical trials. It is conducted during the preclinical stage of drug or chemical development.
2. Why is early toxicity testing important?
Ans:- It helps identify dangerous compounds early, saving time and money. It also improves safety, reduces late-stage drug failures, and protects human participants from harm.
3. What are the common methods used in early toxicity testing?
Ans:- Common methods include in-vitro (cell-based), in-vivo (animal models), and in-silico (computer-based) techniques. Organ-on-chip models are also emerging as a new method.
4. Is early toxicity testing required by regulatory authorities?
Ans:- Yes, agencies like the FDA, EMA, and OECD require early toxicity data to approve investigational new drug (IND) applications or chemical registration.
5. What are the ethical concerns in early toxicity testing?
Ans:- Animal testing raises ethical issues. As a result, alternative approaches like in-vitro and in-silico models are being adopted to reduce animal use.
6. What is the Early Toxicity Testing Market?
Ans:- It refers to the industry providing tools, services, and platforms for preclinical toxicology testing in sectors like pharmaceuticals, cosmetics, chemicals, and food safety.
7. What is driving growth in this market?
Ans:- Factors include rising drug development costs, increased demand for safer products, stricter regulations, and advancements in alternative testing technologies.
8. Which industries use early toxicity testing the most?
Ans:- The pharmaceutical industry leads, followed by cosmetics, food and beverage, chemicals, and environmental testing sectors.
9. What are the major market segments in early toxicity testing?
Ans:- Segments include type (in-vitro, in-vivo, in-silico), application (pharmaceuticals, diagnostics, cosmetics), and end users (CROs, academic labs, biotech firms).
10. Which regions dominate the market?
Ans:- North America holds the largest share due to strong R&D investment and supportive regulations. Europe and Asia-Pacific are also showing fast growth.
Conclusion
The Early Toxicity Testing Market is experiencing steady growth, driven by rising demand for safer, faster, and more ethical drug and chemical screening methods. With a projected market value of US$ 2.7 billion by 2034 and a CAGR of 6.8%, the sector is transforming through innovations like in-vitro assays, organ-on-a-chip systems, and in-silico modeling.
Regulatory bodies such as the FDA and EMA are increasingly supporting these alternatives, helping reduce animal testing and improve predictive accuracy. North America continues to lead, backed by strong pharmaceutical infrastructure and regulatory clarity. As personalized medicine, environmental safety, and product development evolve, early toxicity testing will remain a cornerstone of global safety assessment strategies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



