Introduction
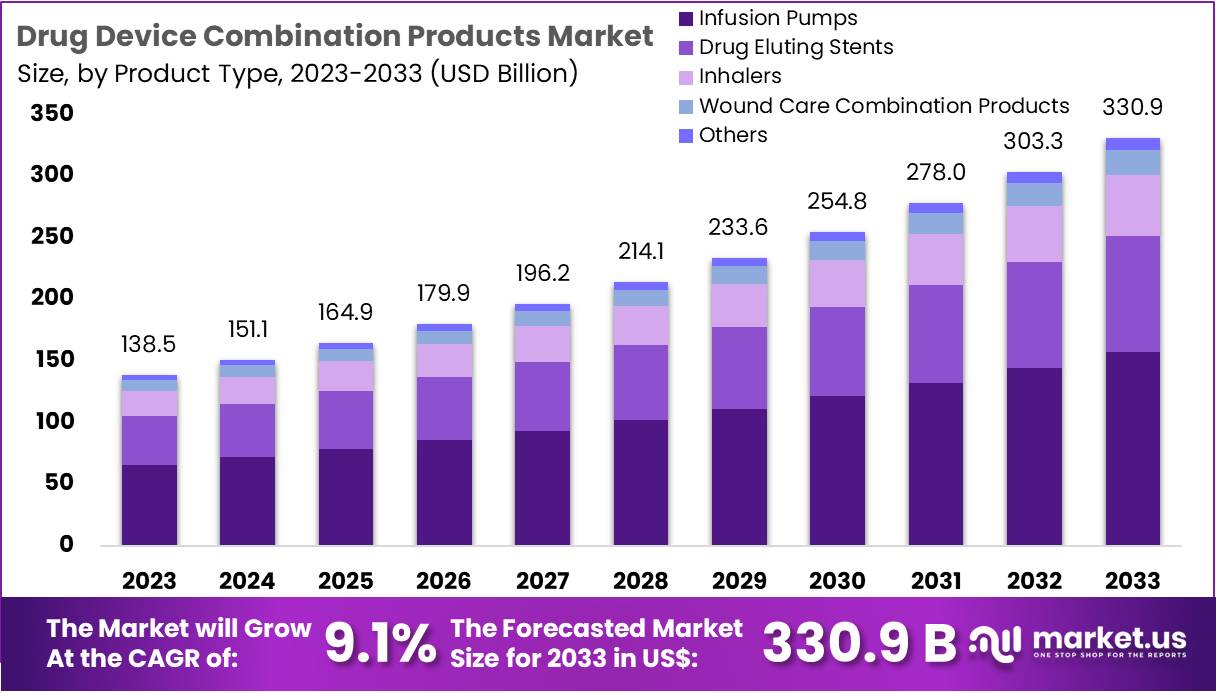
The Drug-Device Combination Products Market is projected to grow significantly, reaching US$ 330.9 billion by 2033 from US$ 138.5 billion in 2023, with a CAGR of 9.1% from 2024 to 2033. This growth is driven by advancements in technology, the increasing prevalence of chronic diseases, supportive regulations, and patient demand for minimally invasive treatments.
Technological innovations in medical devices play a key role in market expansion. For example, Johnson & Johnson’s Varipulse, a pulsed field ablation system approved by the FDA in 2024, highlights the potential of advanced combination products to improve therapeutic outcomes and address unmet medical needs.
The rising burden of chronic conditions such as cardiovascular diseases and diabetes also fuels demand. Drug-eluting stents and insulin infusion pumps provide targeted therapies that enhance patient care and improve quality of life, making them indispensable in modern healthcare.
Regulatory support from agencies like the FDA facilitates product development and approval, encouraging innovation. Additionally, growing patient preference for minimally invasive treatments, such as transdermal patches and inhalers, accelerates adoption. These products offer quicker recovery times and reduced hospital stays, aligning with patient needs and healthcare trends.
With technological progress, regulatory clarity, and changing patient preferences, the drug-device combination products market is poised for sustained growth, offering innovative solutions to enhance global healthcare.
Key Takeaways
- In 2023, the drug-device combination products market generated US$ 138.5 billion, projected to grow to US$ 330.9 billion by 2033 with a 9.1% CAGR.
- Infusion pumps led the product type segment in 2023, accounting for a dominant 47.6% of the market share.
- Diabetes was the leading application, capturing a significant 48.5% share in the drug-device combination products market.
- Hospitals dominated the end-user segment, holding the largest revenue share of 58.1% in 2023.
- North America led the regional market in 2023, commanding a notable 42.5% of the global market share.
Regional Analysis
North America leads the drug-device combination products market with a 42.5% revenue share. Advancements in medical technology and demand for integrated healthcare solutions drive this growth. The aging population and the prevalence of chronic diseases further boost demand for minimally invasive treatments. In February 2023, Teleflex Incorporated introduced the GuideLiner Coast Catheter and Triumph Catheter, highlighting innovation in the region. Products like injectable devices and drug-eluting stents enhance drug delivery, improving patient compliance and treatment outcomes in North America.
The favorable regulatory environment and continuous advancements in combination therapies have strengthened North America’s market position. Increasing adoption of therapies targeting cardiovascular diseases and cancer supports market expansion. The region’s healthcare focus is shifting toward personalized and integrated treatment options. These changes align with the demand for more efficient and patient-friendly solutions. As healthcare systems embrace these innovations, the drug-device combination products market in North America is projected to grow steadily in the coming years.
Asia Pacific is set to witness the highest CAGR during the forecast period due to its expanding healthcare infrastructure. Rising healthcare expenditure and improved patient awareness are major growth drivers. In May 2024, Abbott launched the XIENCE Sierra drug-eluting stent system in India, emphasizing the adoption of advanced combination products. The increasing prevalence of chronic conditions like cardiovascular diseases and diabetes further drives demand. Healthcare access improvements in countries like India and China contribute significantly to market expansion.
Governments in Asia Pacific are investing heavily in enhancing healthcare systems and expanding access to advanced medical technologies. This trend bolsters demand for drug-device combination products. Emerging markets, including Southeast Asia, are adopting sophisticated solutions for disease treatment and management. As awareness grows and healthcare providers integrate advanced technologies, the region is poised for rapid market expansion. The increasing adoption of innovative therapies reflects Asia Pacific’s commitment to improving patient care and driving healthcare innovation.
Emerging Trends
- Technological Advancements: Smarter drug-device combinations are reshaping healthcare. Innovations like injector pens deliver precise medication doses, ensuring better treatment outcomes. These devices improve patient adherence by simplifying medication routines. Advanced technology also enhances safety, reducing the risk of dosing errors. This trend supports personalized medicine, allowing healthcare providers to tailor treatments to individual needs. The focus on precision is driving significant improvements in patient care and satisfaction.
- Increased Demand for Minimally Invasive Devices: Patients increasingly prefer minimally invasive drug delivery methods. Devices such as transdermal patches and inhalers provide painless administration options. These methods enhance comfort and reduce the fear associated with needles. Additionally, they are more user-friendly, making them ideal for self-administration. This trend is gaining traction, especially among patients managing chronic conditions. It highlights the shift towards convenience and better healthcare experiences.
- Integration of Digital Health Technologies: Digital technologies are revolutionizing drug delivery systems. For instance, sensors in inhalers allow real-time monitoring of medication usage. This helps track adherence and ensures patients follow prescribed treatments. Such features also enable personalized healthcare, as data insights help refine treatment plans. The integration of digital tools supports proactive healthcare management, empowering both patients and providers. This trend is expected to drive better clinical outcomes.
- Regulatory Support and Expedited Approvals: Regulatory agencies are simplifying approval processes for combination products. These streamlined pathways encourage innovation and speed up market entry. Faster approvals benefit patients by providing quicker access to advanced treatments. Agencies also promote collaboration between manufacturers and regulators, fostering a supportive environment for research and development. This regulatory push is vital for advancing drug-device combinations and improving patient care worldwide.
Use Cases
- Insulin Injector Pens: Insulin injector pens are designed to simplify diabetes management. These devices combine insulin medication with a pen-like injector, making it easy for patients to administer precise doses. They are user-friendly and highly portable, offering convenience for daily use. Studies reveal that these pens improve patient adherence to insulin therapy, which directly enhances blood sugar control. By ensuring accurate dosing and ease of use, insulin injector pens have become an essential tool in diabetes care.
- Drug-Eluting Stents: Drug-eluting stents are widely used in treating coronary artery disease. These stents are coated with medication that prevents the re-narrowing of arteries after they are widened. Compared to bare-metal stents, they significantly reduce the need for repeat procedures. Their innovative design helps maintain blood flow, lowering the risk of future cardiac events. Drug-eluting stents represent a major advancement in cardiovascular treatment, ensuring better patient outcomes.
- Inhalers with Medications: Inhalers are essential devices for managing respiratory conditions like asthma and COPD. Metered-dose inhalers deliver specific amounts of medication directly to the lungs, ensuring fast and effective relief. These devices are compact and easy to use, making them ideal for daily treatment. Patients experience improved breathing and reduced symptoms when using these inhalers. Their precision and convenience make inhalers a critical tool in respiratory care.
Conclusion
The Drug-Device Combination Products Market is set for remarkable growth, driven by advancements in technology, increasing demand for minimally invasive solutions, and supportive regulatory frameworks. These products address critical healthcare needs by combining drugs with innovative delivery systems, improving patient outcomes and treatment efficiency. Rising chronic disease prevalence and advancements in digital health technologies further bolster their adoption. Key trends, such as personalized medicine and the integration of smart technologies, highlight the market’s evolution. With expanding healthcare access, particularly in Asia Pacific, and continuous innovation, the market holds immense potential to transform global healthcare. This trajectory positions drug-device combinations as a cornerstone of modern, patient-centered medical solutions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



