Table of Contents
Introduction
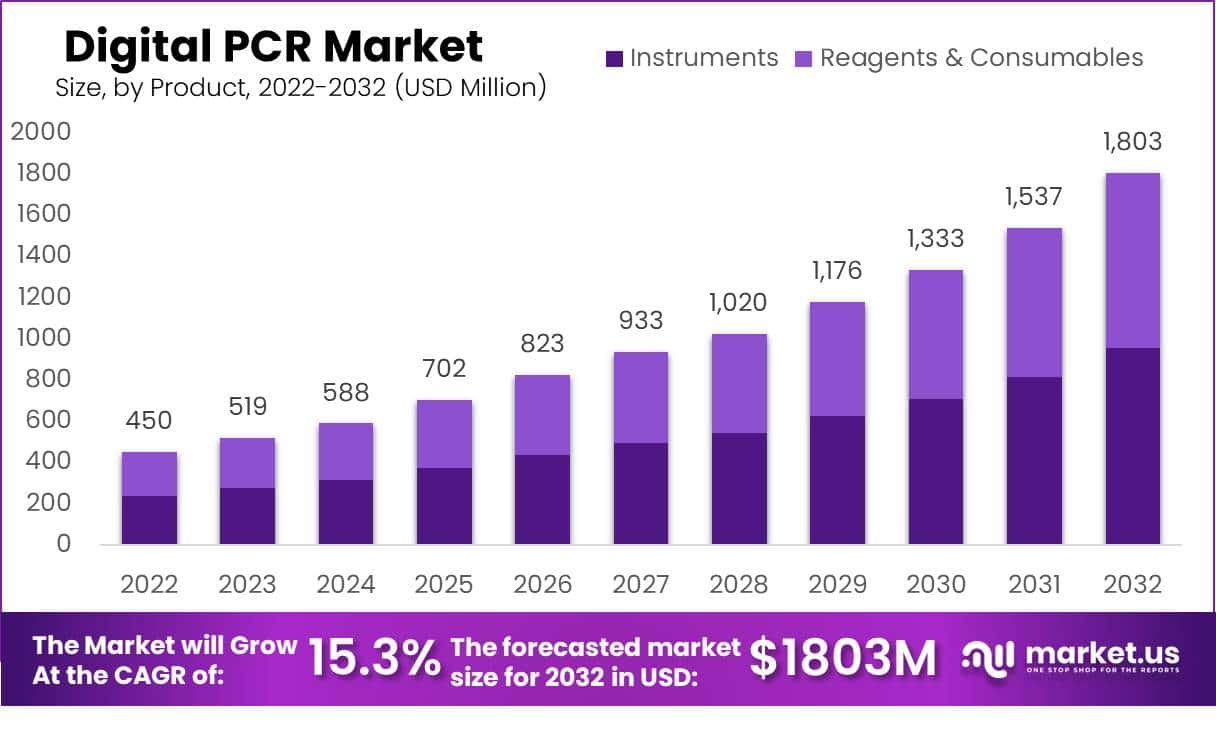
The global Digital PCR market is projected to expand from USD 450 million in 2022 to approximately USD 1,803 million by 2032, achieving a compound annual growth rate (CAGR) of 15.3% during the forecast period from 2023 to 2032. This growth is largely fueled by technological enhancements, increased utilization in clinical diagnostics, and a focus on precision in genomic research. Digital PCR technology has evolved to provide superior accuracy and precision over traditional PCR methods, with innovations in primer design enhancing the specificity and reliability of genetic sequencing in both research and clinical contexts.
In clinical diagnostics, digital PCR’s sensitivity proves critical in detecting low levels of pathogens, genetic mutations, and other target molecules, facilitating early diagnosis and treatment decisions. Its application is particularly vital in identifying cancer biomarkers and genetic disorders, where precise DNA quantification is crucial. Additionally, the technology’s high resistance to inhibitors makes it a preferred choice in research sectors such as environmental monitoring, forensic analysis, and quality control in food products, further driving its adoption.
Educational and government initiatives also support the growth of the digital PCR sector. Courses focusing on digital health and biotechnology are equipping a new generation of researchers and clinicians with necessary skills, while government funding boosts genomic and medical research. This institutional backing ensures a steady advancement in the application and development of digital PCR technologies, reinforcing its integration into modern molecular biology frameworks.
Recent product launches illustrate the sector’s innovation. In September 2024, QIAGEN introduced the PAXgene Urine Liquid Biopsy Set, designed for reliable cell-free DNA analysis from urine samples, enhancing disease monitoring and treatment response. Stilla Technologies, in June 2024, expanded their Nio™ product line with new configurations to accommodate varying laboratory needs, receiving acclaim for design and innovation. Moreover, in February 2023, Thermo Fisher launched the QuantStudio Absolute Q AutoRun dPCR Suite, integrating digital PCR technology with lab automation for high-throughput, multiplex analysis, which streamlines operations and accelerates therapeutic development.
These strategic developments underscore the dynamic nature of the digital PCR market, highlighting its critical role in pushing the boundaries of genomic testing and molecular research. As the technology continues to mature, its applications are set to widen, promising significant impacts on clinical diagnostics, research methodologies, and therapeutic approaches, ensuring robust market growth in the coming years.
Key Takeaways
- Instruments constituted 34% of the total revenue among various product categories in 2022.
- Digital droplet PCR technology claims the largest market share currently.
- Genetic disorders are leading the market, with the highest compound annual growth rate projected from 2023 to 2032.
- Academic and research organizations are the predominant end-users, capturing the largest market share.
- North America generated the highest revenue, accounting for 42% of the market.
- Europe is projected to maintain consistent compound annual growth from 2023 to 2032.
Digital PCR Statistics
- 2022: Total market size of $450 million, with instruments and reagents & consumables combined.
- 2023: Market size increases to $519 million.
- 2024: Further growth, reaching $588 million.
- 2025: Market size expands to $702 million.
- 2026: Significant rise to $823 million.
- 2027: Market size is $933 million.
- 2028: Crosses the billion mark at $1,020 million.
- 2029: Grows to $1,176 million.
- 2030: Size is $1,333 million.
- 2031: Market approaches $1,537 million.
- 2032: Reaches $1,803 million, showcasing robust growth.
Emerging Trends
- Automation and Artificial Intelligence Integration: Digital PCR laboratories are increasingly leveraging artificial intelligence (AI) to enhance operational efficiency. AI technologies streamline the analysis by automating the dPCR process, which significantly reduces the human effort required, accelerates data processing, and improves result accuracy. This automation supports faster and more accurate diagnoses, leading to tailored treatment plans for patients. The integration of AI into dPCR exemplifies the blend of biotechnology with digital advancements, setting a new standard for precision in medical diagnostics.
- Multiplexed Testing Advances: The COVID-19 pandemic has accelerated the adoption of multiplexed digital PCR testing. This method enables the simultaneous detection of multiple pathogens in one test, which is especially beneficial during flu seasons or when differentiating between similar viral infections. Multiplexed testing enhances both the efficiency and the breadth of infectious disease diagnostics, demonstrating the adaptability of dPCR technology in response to global health challenges.
- Broadening Applications Beyond Infectious Diseases: The use of digital PCR is expanding to include a wider range of medical conditions beyond infectious diseases. This includes the detection of genetic disorders, identification of cancer markers, and applications in personalized medicine. The inherent high sensitivity and specificity of dPCR make it ideal for detecting low-level targets within complex biological samples, paving the way for advancements in genetic analysis and cancer research.
- Global Infrastructure Development: Initially driven by the surge in COVID-19 testing, the global infrastructure for digital PCR has seen significant growth. This expansion is not just enhancing the capacity for pandemic response but also strengthening the foundation for ongoing research and application across various medical fields. As the infrastructure continues to develop, it supports a broader application of dPCR, thereby enhancing diagnostic capabilities worldwide and contributing to improved global health outcomes.
Use Cases
- Infectious Disease Diagnosis: Digital PCR (dPCR) is a vital tool for the rapid and accurate identification of pathogens, particularly during disease outbreaks. This technique is pivotal in detecting viral infections and plays a critical role in guiding response strategies. By offering precise quantification and detection capabilities, dPCR enables healthcare professionals to implement effective containment and treatment measures quickly. Its reliability and speed are essential in managing public health crises, ensuring that interventions are based on solid diagnostic evidence.
- Cancer Research and Management: In the realm of oncology, dPCR serves as an advanced method for detecting and quantifying genetic variations and mutations indicative of cancer. This technology facilitates early diagnosis and helps monitor the effectiveness of treatments. By analyzing tumor dynamics in detail, dPCR provides invaluable insights that are crucial for optimizing patient care and managing the disease more effectively. Its ability to detect minute changes in DNA makes it an indispensable tool in the continuous effort to understand and combat cancer.
- Genetic and Prenatal Testing: Digital PCR is renowned for its high sensitivity, making it exceptionally useful in genetic screenings and prenatal diagnostics. It is especially effective in non-invasive prenatal testing, where fetal DNA is examined through maternal blood samples. This application allows for the early detection of genetic disorders, offering expectant parents critical information without the risks associated with invasive test methods. The precision of dPCR ensures reliable results even from minimal DNA samples, enhancing the safety and effectiveness of genetic testing.
- Food Safety and Environmental Monitoring: The application of dPCR extends beyond clinical settings into food safety and environmental monitoring. It is employed to test food products for genetic modifications and pathogens, ensuring compliance with health regulations. Additionally, dPCR is used to detect microorganisms or genetic materials in environmental samples from water and soil. This capability is crucial for monitoring pollution levels and conducting ecological studies, helping maintain ecosystem health and public safety. By ensuring the accuracy of these tests, dPCR plays a critical role in upholding environmental and food safety standards.
Key Players Analysis
QIAGEN
QIAGEN has been making significant advancements in the Digital PCR (dPCR) sector, particularly with their QIAcuity platform. In 2024, they launched over 100 new assays designed for a variety of applications, including cancer research, inherited genetic disorders, infectious disease surveillance, and more. These assays are part of QIAGEN’s GeneGlobe platform, which already boasts more than 2,300 validated assays. This expansion highlights QIAGEN’s response to the growing need for precise and sensitive genetic detection tools in both research and clinical settings.
The QIAcuity platform itself is noted for its efficiency and precision, capable of quantifying low-abundance DNA and RNA thanks to its innovative use of nanoplates that segment a sample into thousands of tiny partitions. This technology not only enhances the sensitivity of genetic analyses but also significantly cuts down processing times from six hours to just two. Looking ahead, QIAGEN is set to launch an in-vitro diagnostic version of this platform, further expanding its clinical applications, such as monitoring cancer progression and diagnosing infectious diseases through less invasive methods like liquid biopsies.
Thermo Fisher Scientific Inc.
Thermo Fisher Scientific Inc. has made significant strides in the digital PCR (dPCR) sector, positioning itself as a leader in precision genetic analysis. The company’s Applied Biosystems QuantStudio Absolute Q Digital PCR System exemplifies their innovation. This system is designed for high accuracy and quick results, making it ideal for applications such as oncology, cell and gene therapy, and other research areas that require precise nucleic acid quantification.
Thermo Fisher’s dPCR solutions are recognized for their ability to deliver reliable and consistent data, which is crucial for the detection and quantification of genetic variations. The system’s design simplifies workflows, reducing the hands-on time to just five minutes and minimizing user error. This has been particularly transformative in accelerating research and development timelines in the biopharmaceutical sector, where it is used for drug discovery and other molecular research applications.
Bio-Rad Laboratories, Inc.
Bio-Rad Laboratories, Inc. is a prominent player in the digital PCR (ddPCR) sector, known for its QX600 Droplet Digital PCR System. This system enhances measurable residual disease research with its ability to perform highly sensitive and multiplexed analyses, enabling the detection of multiple genetic targets within a single well.
It’s designed for ease of use and compatibility with existing protocols, making it an efficient choice for clinical research. The system is part of Bio-Rad’s broader portfolio that includes other advanced platforms like the QX200 and QXDx systems, catering to various research needs.
JN Medsys
JN Medsys is a prominent player in the digital PCR (dPCR) market, specializing in the development of genomic tools and diagnostic kits for nucleic acid analysis. Their flagship product, the Clarity™ digital PCR system, is renowned for its precision, accuracy, and sensitivity, making it a valuable tool for researchers and clinicians.
This system distinguishes itself with a unique chip-in-a-tube design, allowing rapid and efficient partitioning of samples into as many as 10,000 discrete sections, facilitating high-resolution quantification of target nucleic acids. This design integrates the robustness of chip-based partitioning with the simplicity of tube-strip usage, making digital PCR processes both faster and more user-friendly.
In recent developments, JN Medsys introduced the Clarity Plus™ digital PCR system, an advancement offering even higher resolution and multiplexing capabilities without compromising workflow efficiency. Capable of handling 96 reactions per run, this system subdivides each sample into 45,000 partitions, enhancing the detection and quantification of multiple targets simultaneously, which is especially beneficial in complex diagnostic scenarios like cancer and infectious diseases detection.
Stilla
Stilla Technologies is actively shaping the landscape of digital PCR with its advanced multiplex technology. This company is distinguished by its groundbreaking innovations in digital PCR systems, including the Nio™+ instrument, which is celebrated for its high throughput, random access, and automation capabilities. This technology allows for the simultaneous quantification of multiple genetic markers, enhancing precision in genetic analysis. Recently, Stilla closed a significant Series C financing of $26.5 million, directed at amplifying their commercial operations, particularly focusing on markets like Biopharma, Applied Testing, and Clinical applications.
A significant component of Stilla’s strategy includes a partnership strategy to boost the commercial viability of their offerings, exemplified by their recent collaboration with Niba Labs to enhance the development of digital PCR assays for cell and gene therapies. This points to Stilla’s commitment to expanding the practical applications of digital PCR technology across various fields including oncology, infectious disease, and environmental testing, thereby setting a high standard in the digital PCR market.
Sysmex Corporation
Sysmex Corporation, a leader in the diagnostics sector, has significantly advanced its capabilities in digital PCR (dPCR) technologies, particularly through its subsidiary, Sysmex Inostics. This subsidiary is renowned for its highly sensitive mutation detection capabilities in oncology, using proprietary technologies such as OncoBEAM™ and SafeSEQ.
For instance, OncoBEAM™ has demonstrated enhanced performance in detecting mutations in circulating tumor DNA, which is critical for non-invasive cancer diagnostics. The technology has shown a two-fold higher detection rate of specific mutations in patients with non-small cell lung cancer (NSCLC) compared to other methods.
Standard BioTools
Standard BioTools is making significant strides in the digital PCR sector with its innovative Biomark X9 System. This system is a versatile, microfluidics-based benchtop platform that excels in delivering high-throughput genomics solutions. Capable of handling thousands of nanoliter-scale reactions in a single run, it provides a cost-effective and scalable solution for comprehensive sample profiling with minimal operator intervention.
The Biomark X9 System enhances laboratory productivity by allowing the generation of up to 46,080 data points per 8-hour shift and the production of up to 384 barcoded libraries daily for next-generation sequencing (NGS) needs. This integration of real-time PCR and NGS library preparation on a single compact platform demonstrates Standard BioTools’ commitment to advancing genomic research through enhanced efficiency and deep insights with nanoscale genomics.
Precigenome LLC.
Precigenome LLC, a company founded in 2017 and based in San Jose, California, has specialized in the field of Digital PCR (dPCR). This technology allows for highly sensitive and accurate measurement of DNA or RNA in samples, making it a fundamental tool for genetic testing and analysis in various sectors, including medical and clinical laboratory research.
Precigenome LLC develops and markets an array of PCR assays and in vitro diagnostics (IVD) systems that leverage microfluidic platforms for applications such as lipid nanoparticle synthesis, droplet generation, and cell isolation. The company’s innovation in the dPCR sector includes various PCR kits like the FastPlex™ series, which are essential for detecting pathogens like SARS-CoV-2 and for genetic testing of diseases such as leukemia
Conclusion
The Digital PCR market is set to experience substantial growth due to its precision and broadening applications in genetic research, clinical diagnostics, and environmental analysis. As technology advances, digital PCR offers unmatched accuracy, making it essential for detecting low-level genetic variations and aiding in disease management and treatment. With continued innovation from key players and increasing global infrastructure, digital PCR is becoming more integrated into medical and research practices. This expansion is expected to drive further technological advancements and enhance its applications, confirming digital PCR’s vital role in advancing molecular diagnostics and research across various sectors.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



