Overview
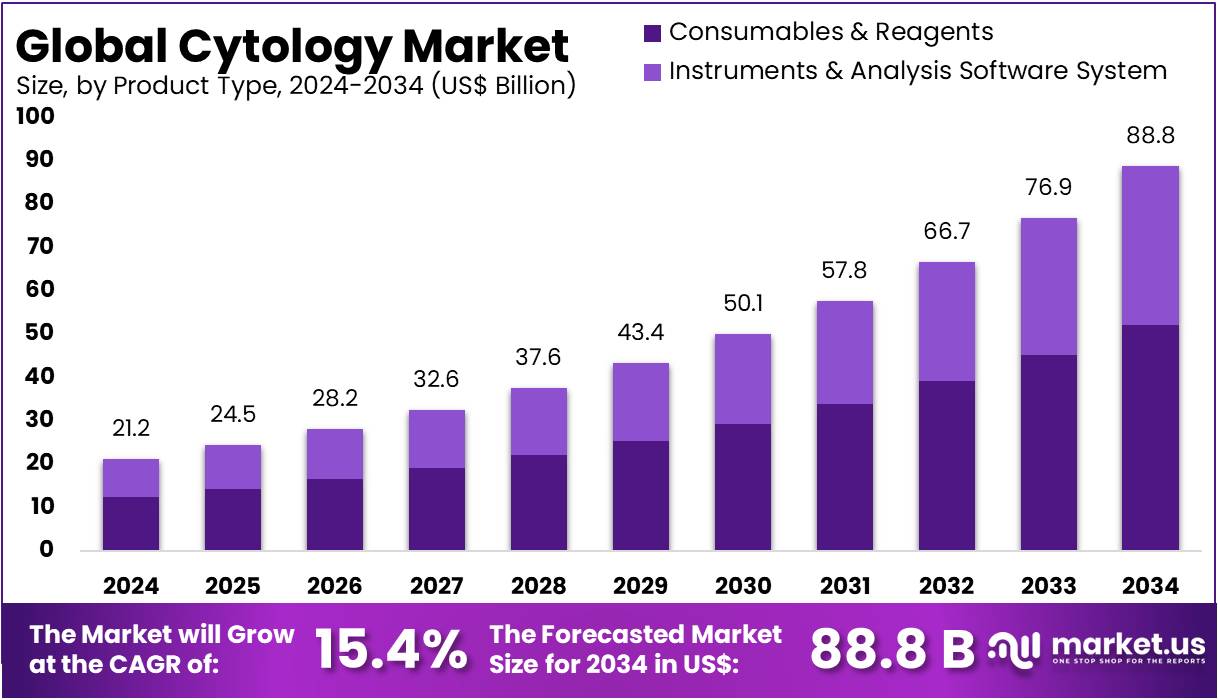
New York, NY – August 19, 2025: The Cytology Market is projected to reach US$ 88.8 billion by 2034, growing from US$ 21.2 billion in 2024. This reflects a strong CAGR of 15.4% between 2025 and 2034. North America leads with a 39.9% market share, valued at US$ 8.5 billion in 2024. Market growth is supported by aging populations, a rising cancer burden, and organized screening programs. These factors ensure steady demand for both gynecologic and non-gynecologic cytology, including triage testing and diagnostic fine-needle aspiration (FNA).
Cancer Burden Driving Demand
According to the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC), global cancer incidence will surpass 35 million new cases by 2050, marking a 77% rise from 2022. This demographic and risk-factor shift underlines the vital role of cytology in screening and diagnosis. Cervical cancer remains a core focus, with 662,301 new cases and 348,874 deaths recorded in 2022. Most of these deaths occur in low- and middle-income countries, where screening access is still limited.
Cervical Screening and HPV Programs
Cytology continues to play an important role in cervical cancer pathways. While HPV testing is becoming the primary screening tool, cytology is essential for triage and follow-up. WHO’s 2024 guidelines endorse HPV nucleic acid tests as the first line of screening, with cytology serving as reflex testing for abnormal cases. National programs, such as the NHS in the UK, already follow this approach. This ensures cytology remains relevant as a diagnostic step, particularly in confirming abnormal or HPV-positive results.
Policy and Participation Initiatives
Global strategies are reinforcing cytology demand. WHO’s initiative to eliminate cervical cancer sets 2030 targets for vaccination, screening, and treatment. New commitments in 2024 and 2025 aim to expand vaccine coverage and screening access, particularly in underserved regions. In the U.S., the FDA authorized self-collected vaginal samples for HPV testing in 2024, a decision expected to boost participation. Increased uptake of self-sampling will generate more HPV-positive cases, each requiring cytology triage and colposcopy referral, creating predictable demand for laboratories.
Role of Vaccination and Screening Balance
Although HPV vaccination is scaling up, cytology remains necessary in the medium term. WHO estimated that global HPV vaccine coverage for girls was only 31% in 2024. Many older women remain unvaccinated, meaning cytology is critical for managing existing infections and cancers. As both vaccination and screening expand, cytology volumes are expected to grow from triage testing and diagnostic confirmations, rather than primary screening, shifting its role but keeping its importance intact.
Technology and Workforce Transformation
Technology adoption is enhancing efficiency in cytology. The FDA has approved automated imaging systems for Pap tests and authorized whole-slide imaging for digital pathology. These advances encourage investments in digital cytology and remote review, increasing throughput and quality. At the same time, a shortage of laboratory professionals is pushing facilities toward automation and standardized workflows. This transition supports faster turnaround times while maintaining accuracy and meeting growing test demand from screening programs.
Beyond Cervical Cancer: Wider Clinical Use
Cytology continues to play a role outside cervical screening. The U.S. National Cancer Institute recommends FNA cytology in diagnosing thyroid nodules and other malignancies. With medical imaging detecting more nodules and masses, minimally invasive cytology is increasingly used for first-line assessment and treatment planning. This application broadens cytology’s value beyond gynecological testing, making it a vital tool in oncology and routine diagnostics, further strengthening its market outlook for the coming decade.
Key Takeaways
- In 2024, the cytology market earned US$ 21.2 billion revenue and is forecasted to reach US$ 88.8 billion by 2034, growing 15.4% CAGR.
- By product type, consumables and reagents dominated in 2023, capturing 58.7% share, surpassing instruments and analysis software systems in the cytology market.
- When segmented by examination type, cytology maintained a significant lead with 62.1% market share, compared to histology’s relatively smaller contribution.
- Within applications, drug discovery and designing stood out as the top segment, accounting for 48.5% of cytology market revenues in 2023.
- Hospitals emerged as the leading end-user segment, holding 52.4% revenue share, ahead of biotechnology, pharmaceuticals, diagnostic centers, and other institutions.
- North America secured the top regional position in 2023, contributing 39.9% of global cytology revenues, driven by advanced healthcare infrastructure and R&D spending.
Regional Analysis
North America held a dominant 39.9% share of the cytology market in 2024. Growth was driven by high demand for cancer screening and advanced diagnostic technologies. Cervical cancer screening played a major role in reducing disease burden. CDC data shows a 79% decline in cervical precancer among US women aged 20–24 from 2008 to 2022. Women aged 25–29 saw a 37% reduction, proving the impact of early detection. These programs continue to strengthen diagnostic adoption across the region.
Rising cancer cases in the US are supporting cytology market expansion. The National Cancer Institute projects 226,650 new lung cancer cases in 2025. Bladder cancer cases are estimated at 84,870, while thyroid cancer may reach 44,020. All rely heavily on cellular analysis for diagnosis. Leading companies have shown strong performance. Hologic’s diagnostics revenue rose 9.2% in Q4 2024, reaching US$ 987.9 million. Danaher reported US$ 23.9 billion revenue, with diagnostics contributing significantly to its business.
The Asia Pacific region is projected to record the highest CAGR in the coming years. A growing and aging population, coupled with rising cancer incidence, is creating strong market demand. Government initiatives are central to this expansion. In China, cervical cancer screening coverage reached 51.5% in 2023–2024. This surpassed the 2025 national target of 50%. The China CDC Weekly confirms this achievement, reflecting strong public health commitment. WHO also supports regional programs for cancer control and workforce training.
Cytology adoption in Asia Pacific is also supported by digital and automated pathology. These tools improve diagnostic accuracy and efficiency. Global players are expanding operations to leverage regional opportunities. Roche Diagnostics supplied 30 billion tests worldwide in 2024, showing its strong contribution to cellular analysis. Becton Dickinson (BD) Life Sciences continues to invest in Asia Pacific, reinforcing market growth. The combination of technology adoption, international partnerships, and healthcare policies will keep this region as the fastest-growing cytology market.
Emerging Trends
- HPV-first screening, cytology as triage: Many countries are moving from Pap smears as the first test to HPV testing as the main screening tool. Cytology is now often used as a reflex or triage step when HPV results come back positive. This change aligns with the World Health Organization’s cervical cancer elimination plan, which aims for 90% HPV vaccination, 70% screening, and 90% treatment by 2030. In 2022, there were around 660,000 new cervical cancer cases and 350,000 deaths worldwide. These numbers show the urgent need for screening programs. Cytology still plays a key role, especially in triaging HPV-positive women.
- Self-collection expands access: In May 2024, the FDA allowed people to collect their own vaginal samples in a health-care setting for two HPV tests: BD Onclarity and Roche cobas. This update is a big step for improving access to cervical cancer screening. Self-collection makes it easier for more people to get tested, especially those who avoid clinic-based screening. However, cytology is still needed in the process. When self-collected samples test positive for HPV, laboratories often use cytology for triage. This ensures that follow-up is accurate, cost-effective, and accessible. The combination of HPV testing and cytology improves overall screening outcomes.
- AI-assisted digital cytology: Artificial intelligence is becoming part of routine cytology practice. In February 2024, Hologic’s Genius™ Digital Diagnostics System became the first FDA-cleared AI-driven cytology system. It uses volumetric imaging and machine learning to identify suspicious cells on ThinPrep Pap slides. The system highlights the most relevant fields of view, allowing cytologists to focus on high-risk areas first. This reduces workload and improves accuracy. Studies cited by the FDA confirm that this tool helps streamline workflow without reducing diagnostic quality. AI in cytology is expected to grow rapidly, making labs more efficient and supporting better cancer detection rates worldwide.
- Telecytology and whole-slide imaging: Telecytology is expanding with the use of whole-slide imaging (WSI). This approach allows cytology slides to be scanned and shared remotely for expert review. The College of American Pathologists (CAP) has published guidelines to validate this process. Telecytology is already helping with rapid on-site evaluation (ROSE) and remote consultations. Smaller laboratories, community hospitals, and outreach programs benefit the most. It allows specialists to review samples without being physically present. This is especially important in underserved regions where access to trained cytologists is limited. With digital platforms improving, telecytology is becoming a trusted and scalable solution.
- ROSE as standard in minimally invasive sampling: Rapid on-site evaluation (ROSE) is becoming a standard part of many minimally invasive sampling procedures. Cytology teams use ROSE during tests such as endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). The purpose is to confirm sample adequacy in real time. Studies show that ROSE achieves around 97% sensitivity and 97% specificity in EBUS-TBNA. This reduces the need for repeat procedures and improves diagnostic yield on the first attempt. Hospitals adopting ROSE report better efficiency and fewer patient recalls, making it a cost-effective and clinically valuable tool.
- Beyond Pap: broader cytology portfolio: Cytology is no longer limited to Pap smears. A wider range of applications is growing across urine, respiratory, pancreatic, and thyroid samples. For example, urine cytology combined with reflex molecular tests such as FISH helps detect bladder cancer. In lung cancer, cytology from EBUS-TBNA remains a frontline diagnostic method. Pancreatic lesions are often sampled through EUS-FNA or fine-needle biopsy. Thyroid nodules also rely on cytology as part of standardized diagnostic pathways. Laboratories are adopting digital tools to improve accuracy and efficiency. This diversification ensures cytology remains relevant beyond cervical cancer screening programs.
- Education and workforce development: The cytology workforce faces increasing pressure due to limited staffing and rising demand. Universities and training centers are now adopting virtual microscopy and whole-slide imaging to address this challenge. Virtual slides allow students and trainees to study real-world cytology cases without needing physical glass slides. This improves access to training, ensures consistent teaching quality, and helps standardize competencies across institutions. Remote learning tools also support continuing education for professionals in smaller labs. By combining digital platforms with traditional teaching, education in cytology is becoming more accessible and future-ready, preparing the next generation of skilled cytologists.
Use Cases
- Cervical Cancer Screening & Triage: Cytology plays a key role in cervical cancer control. It is still used as a primary Pap test in some regions, but more often serves as triage for women who test HPV-positive. Cytology helps prevent progression by identifying at-risk women early. In 2024, the FDA approved self-collection for HPV tests by Roche and BD, expanding screening access. AI-assisted digital cytology also gained FDA clearance, speeding review and highlighting abnormal cells while keeping diagnostic accuracy high.
- Lung Cancer Diagnosis & Staging (EBUS-TBNA Cytology): For lung cancer, cytology from endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) is highly effective. This minimally invasive procedure samples mediastinal lymph nodes and lung masses. Studies consistently show sensitivity around 90% and specificity close to 100% in experienced centers. Rapid on-site cytology evaluation (ROSE) further boosts efficiency. In one study, ROSE reached 97.4% sensitivity and 96.9% specificity. This reduces the need for repeat procedures, improving patient care and hospital workflow. With lung cancer being the leading cause of cancer death globally, EBUS-TBNA cytology has become essential for accurate diagnosis and staging.
- Pancreatic Lesions (EUS-FNA/EUS-FNB Cytology): Cytology also supports diagnosis of pancreatic lesions. Endoscopic ultrasound–guided fine-needle aspiration (EUS-FNA) or fine-needle biopsy (EUS-FNB) provides tissue from pancreatic masses and cysts. Meta-analyses report sensitivity between 79–87% and specificity ranging from 76% to above 90% for malignancy. Newer biopsy needles and techniques like macroscopic on-site evaluation (MOSE) or rapid on-site evaluation (ROSE) improve yield. This reduces inconclusive results and speeds patient management. Given pancreatic cancer’s aggressive nature and poor survival rates, accurate cytology-guided diagnosis is critical for timely treatment decisions and better clinical outcomes.
- Bladder Cancer Detection (Urine Cytology ± FISH): Urine cytology is a widely used, non-invasive test for bladder cancer detection and surveillance. It offers excellent specificity, about 97% in one study with 1,835 patients, but sensitivity remains low at around 29%. To improve detection, labs often combine cytology with UroVysion FISH testing. FISH raises sensitivity to about 62% while maintaining specificity near 90%. Another trial reported sensitivity of ~69% for cytology and ~67% for FISH, though specificity was lower at 72–76%. By combining both, clinicians can balance accuracy and reduce missed cancers, especially in patients with recurrent or high-risk disease.
- Telecytology & Remote Triage: Telecytology is transforming access to cytology expertise. It allows remote review of slides for HPV-positive triage and supports rapid on-site evaluation (ROSE) in facilities without a cytopathologist. Whole-slide imaging (WSI) technology has proven reliable, with pilot studies showing strong diagnostic concordance. This approach offers faster feedback, wider coverage, and improved workflow efficiency. It also supports self-collection programs by enabling remote analysis of HPV-positive samples. With global shortages of trained cytologists, telecytology is a practical solution to extend diagnostic services and maintain quality in both urban hospitals and rural or resource-limited settings.
- Digital & AI Cytology: AI-driven cytology is enhancing accuracy and efficiency in labs. Digital slide scanning with AI pre-screening helps identify abnormal fields faster, reduces manual workload, and allows remote reads. In 2024, the FDA cleared the first AI-powered digital cytology system for Pap slides, a milestone for cervical cancer screening. These systems improve consistency, cut review times, and help cytopathologists focus on high-risk cases. Digital workflows also enable better archiving and data sharing across centers. By combining cytology expertise with AI, labs can handle increasing screening demands while maintaining quality and speed.
Conclusion
The cytology market is entering a phase of steady and long-term growth, supported by global cancer trends, screening programs, and technological advancements. While HPV testing is becoming the main tool for cervical screening, cytology remains essential for triage, follow-up, and diagnosis across different cancers. The rise of digital tools, automation, and AI is making laboratories faster and more accurate, while telecytology is helping extend access to underserved areas. Beyond cervical cancer, cytology plays a key role in diagnosing lung, bladder, thyroid, and pancreatic diseases. With strong policy support, growing awareness, and wider clinical applications, cytology is expected to stay relevant and vital for modern healthcare systems.
View More
Flow Cytometry Market || Immune Thrombocytopenia Market || Histology and Cytology Market || Cytotoxic Drugs Market || Indolent Systemic Mastocytosis Treatment Market || Molecular Cytogenetics Market || Cytology Market || Molecular Diagnostics Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



