Table of Contents
Overview
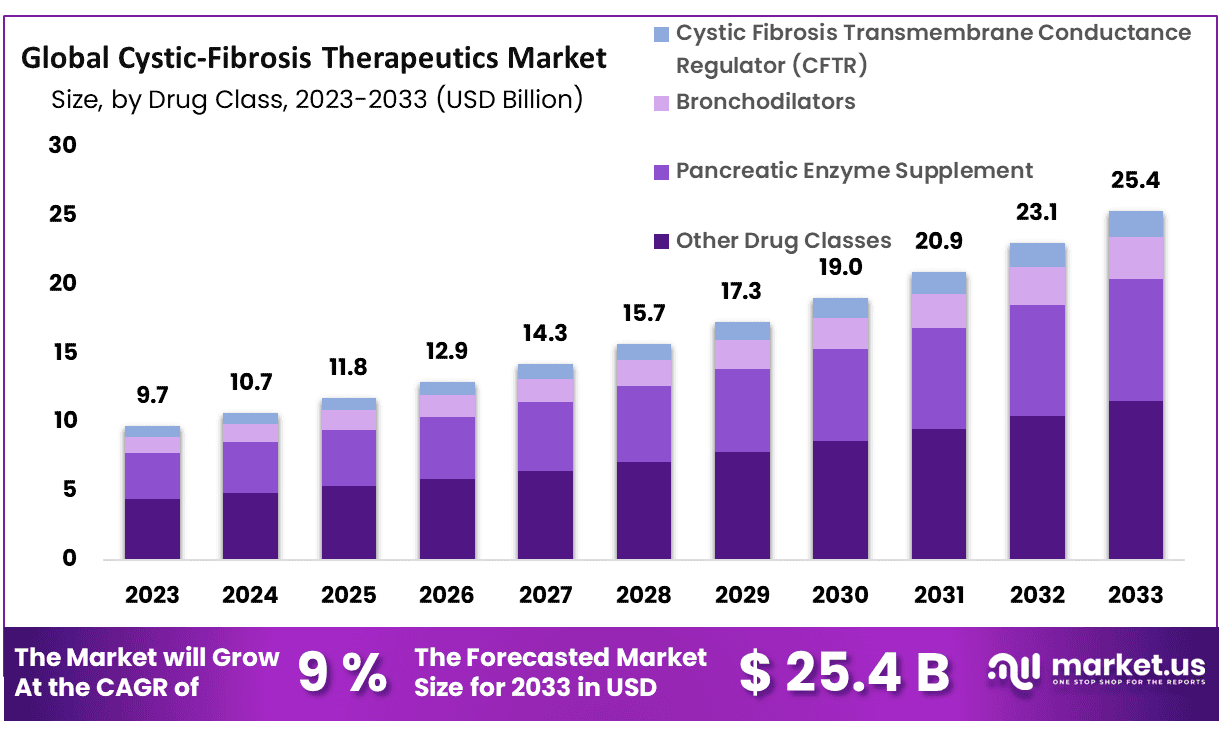
New York, NY – Oct 27, 2025 – The Global Cystic-Fibrosis Therapeutics Market size is expected to be worth around USD 25.4 Billion by 2033 from USD 10.7 Billion in 2024, growing at a CAGR of 9.0% during the forecast period from 2025 to 2033.
The global cystic fibrosis (CF) therapeutics market is experiencing consistent growth driven by increasing disease prevalence, enhanced diagnostic capabilities, and accelerated advancements in targeted therapies. Cystic fibrosis is recognized as a life-threatening genetic disorder that affects nearly 90,000 individuals worldwide. The rise in patient awareness and improved newborn screening programs have expanded the diagnosed patient pool, which continues to support market expansion.
Revenue growth is strongly supported by the adoption of CFTR modulator therapies, which address the underlying genetic mutation rather than merely managing symptoms. The introduction of innovative combination drugs has significantly improved patient outcomes, increasing life expectancy and treatment compliance. The market value is expected to rise steadily as regulatory approvals for novel therapeutics continue to progress in key regions.
North America retains the largest market share due to well-established healthcare systems and supportive reimbursement frameworks. Meanwhile, the Asia-Pacific region is projected to exhibit the highest growth rate, attributed to increased healthcare investments and improving genetic testing infrastructure.
Research collaborations between biotechnology firms, pharmaceutical companies, and academic institutions have strengthened the clinical pipeline, enabling continuous development of advanced treatments, including gene therapy and mRNA-based modalities. The growth of the market can be attributed to these strategic initiatives and favorable government policies that encourage rare-disease drug development.
The cystic fibrosis therapeutics market is anticipated to maintain a positive trajectory, driven by innovation, high unmet clinical needs, and expanding treatment accessibility across emerging economies.
Key Takeaways
- Market Size: The cystic fibrosis therapeutics market is projected to reach USD 25.4 billion by 2033, advancing from USD 10.7 billion in 2024, signifying strong long-term value creation opportunities.
- Market Growth: Sustained expansion is indicated, with the market expected to grow at a CAGR of 9.0% during the forecast period of 2024–2033.
- Drug Class Analysis: CFTR modulators accounted for the largest share of the market, representing 45.4% of total revenue in 2023, driven by increased adoption of high-efficacy targeted therapies.
- Route of Administration Analysis: The oral segment remained dominant, capturing 62% market share, supported by patient convenience and improved adherence rates.
- Distribution Channel Analysis: Hospital pharmacies led the distribution landscape with a 34% market share, owing to strong access to advanced therapeutics and specialist care.
- Regional Insights: North America emerged as the leading region with a 42% revenue share, generating over USD 1 billion due to well-established healthcare infrastructure and favorable reimbursement policies.
- Personalized Medicine: Broader access to genetic testing is advancing precision-based therapies, reinforcing the shift toward personalized medication approaches for CF patients.
- Innovative Research: Active research initiatives by leading biopharmaceutical companies, including Vertex Pharmaceuticals and Novartis, continue to drive the development of next-generation treatment options.
- Global Opportunity: Expansion into emerging markets, coupled with a strong clinical pipeline, is expected to introduce improved outcomes and widen treatment access.
- Patient-Centric Care: Greater emphasis on telehealth and remote monitoring solutions is improving disease management and enhancing patient quality of life.
Regional Analysis
The global cystic fibrosis therapeutics market can be segmented regionally into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. In 2023, North America emerged as the most dominant region, capturing 42% of the global market share and generating approximately USD 1 billion in revenue.
The significant disease burden, particularly among the Caucasian population, along with strong advocacy and support networks such as Cystic Fibrosis Canada and the Cystic Fibrosis Foundation, continue to drive early diagnosis and widespread access to advanced therapies in the region.
The Asia-Pacific region is forecast to be the fastest-growing market, with an anticipated CAGR of 19.4% during the study period. Although the prevalence of cystic fibrosis remains comparatively lower due to underdiagnosis and limited national patient registries, rising disease awareness and advancements in healthcare infrastructure are expected to accelerate therapeutic adoption. Increasing economic development and improving availability of specialized genetic testing and treatment facilities will further contribute to regional expansion.
Across global markets, growth will be supported by rising awareness among healthcare providers and patients regarding innovative therapeutic options. Despite the current limited availability of CF-specific drugs in several regions, an expanding product pipeline, regulatory approvals, and new drug launches are expected to significantly enhance treatment accessibility and stimulate market growth over the forecast period.
Emerging Trends
- Advancements in CFTR Modulator Therapies
The introduction of highly effective CFTR modulator therapies (HEMTs) has transformed the treatment landscape for cystic fibrosis. The approval of the triple-combination therapy Trikafta represented a major advancement, with eligibility covering approximately 90% of the global CF patient population. These therapies improve the function of defective CFTR proteins, resulting in enhanced lung performance, reduced exacerbations, and improved quality of life. - Progress in Gene Editing and Gene Therapy
Innovative genetic interventions, including CRISPR-Cas9-based approaches, are being developed to correct disease-causing CFTR mutations. Research involving patient-derived cells has demonstrated successful gene correction, indicating the possibility of durable or permanent therapeutic outcomes. These advancements signify a fundamental shift from symptomatic management to targeting the underlying genetic defect. - Bacteriophage-Based Interventions for Chronic Infection
Persistent pulmonary infections, particularly caused by Pseudomonas aeruginosa, remain a major clinical burden in cystic fibrosis. Clinical studies supported by the NIH are evaluating bacteriophage therapy as a precision antibacterial strategy. By selectively killing pathogenic bacteria, bacteriophages offer a promising option for individuals with antibiotic-resistant infections.
Use Cases
- CFTR Modulators Improving Pulmonary Outcomes
CFTR modulators such as ivacaftor and lumacaftor have demonstrated measurable improvements in lung function. Clinical data show increases of approximately 10% in forced expiratory volume in one second (FEV₁) within weeks of therapy initiation. These improvements are associated with enhanced respiratory capacity and reduced hospitalization frequency. - Gene Therapy Demonstrating Early Clinical Efficacy
Phase I/II clinical programs are actively evaluating gene delivery strategies to reinstate normal CFTR function in airway tissues. Initial findings indicate partial functional restoration, with up to 25% CFTR correction achieved in treated cells. These results support the long-term potential of gene therapy as a disease-modifying treatment. - Bacteriophage Therapy Addressing Drug-Resistance
For patients with drug-resistant Pseudomonas infections, bacteriophage therapy provides a targeted antimicrobial alternative. Early clinical observations have shown decreases in bacterial burden and measurable respiratory improvements. As a complementary modality, this approach is being positioned to enhance infection management in advanced disease states.
Frequently Asked Questions on Cystic-Fibrosis Therapeutics
- What causes cystic fibrosis and why do patients need specialized drugs?
Cystic fibrosis is caused by mutations in the CFTR gene, leading to thick mucus production that damages organs. Specialized therapeutics target this genetic defect and manage complications, improving respiratory function and overall patient quality of life. - What key drug classes are used in cystic fibrosis treatment?
Drug classes used include CFTR modulators, mucus thinners, antibiotics, bronchodilators, and pancreatic enzyme supplements. Their combined use addresses both genetic dysfunction and respiratory and digestive complications seen in cystic fibrosis patients. - How do CFTR modulators improve patient outcomes?
CFTR modulators correct the underlying chloride channel defects in cystic fibrosis. These therapies enhance lung performance, reduce hospitalization rates, and support longer life expectancy compared to standard symptom-management treatment alone. - Are there ongoing research and development activities for new therapies?
Active research focuses on precision medicine, gene editing technologies, and advanced CFTR modulators. These innovations aim to provide stronger therapeutic responses and expand treatment access to patients with rare CFTR mutations. - Which factors drive market growth?
Market growth can be attributed to rising diagnosis rates, better newborn screening programs, and advances in disease-modifying drugs. Strategic investments by pharmaceutical companies strongly support commercialization of breakthrough CF therapies. - Which regions hold the dominant market share?
North America holds the leading share due to established healthcare infrastructure and early access to high-cost therapies. Europe follows closely, while Asia-Pacific shows improving growth as diagnostic and treatment capabilities expand. - Who are the leading companies in this sector?
Key market participants include Vertex Pharmaceuticals, AbbVie, and F. Hoffmann-La Roche. Their extensive product pipelines, strategic collaborations, and sustained R&D investment strengthen their competitive positions in cystic fibrosis therapeutics.
Conclusion
The global cystic fibrosis therapeutics market is expected to maintain a strong growth trajectory due to rising diagnosis, increased availability of targeted therapies, and expanding patient access across developed and emerging regions. Significant therapeutic progress driven by CFTR modulators, gene therapies, and infection-focused innovations continues to improve patient outcomes and survival rates.
Supportive reimbursement frameworks, strategic research collaborations, and government incentives promote further product development and commercialization. Although high treatment costs and limited accessibility in certain countries remain challenges, the increasing clinical pipeline and enhancement in healthcare infrastructure are expected to strengthen long-term market prospects and deliver better quality of life for CF patients worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



