Table of Contents
Introduction
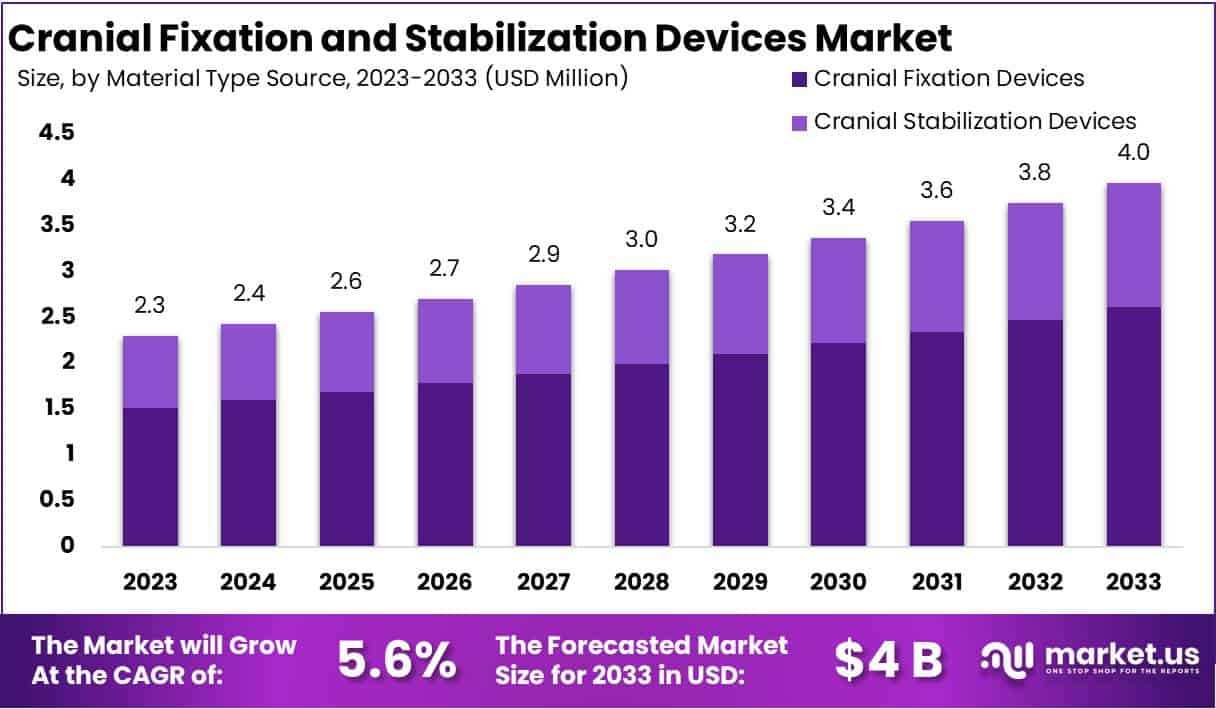
The Global Cranial Fixation and Stabilization Devices Market is projected to expand significantly, with an anticipated growth from USD 2.3 Billion in 2023 to around USD 4 Billion by 2033, marking a CAGR of 5.6%. This growth is fueled by several key factors including technological advancements, demographic shifts, and regulatory progress.
Technological innovations such as the Dynamic Stabilization System (DSS) offer flexible solutions for spinal stabilization, catering to conditions like lumbar spine disorders. These systems permit controlled spinal movements, maintaining stability and preventing discomfort or further damage. Additionally, the integration of materials like titanium and biocompatible coatings in screws and cages enhances device effectiveness and patient outcomes in cranial surgeries.
The increasing global elderly population contributes significantly to the market growth, raising the demand for advanced and minimally invasive surgical interventions. Devices such as the BAK™ and Ray Threaded Fusion Cage™, which have received FDA approval, demonstrate the impact of regulatory endorsements on device adoption and market trust.
Support from government and insurance bodies also plays a crucial role. For example, Medicare Part D helps manage medical device costs, making advanced technologies more accessible and affordable. Furthermore, ongoing education and training initiatives ensure that healthcare professionals are adept at employing these innovative cranial fixation techniques, boosting their widespread adoption.
Recent mergers and product launches further illustrate the dynamic nature of this market. In October 2023, KLS Martin’s merger with KARL STORZ SE & Co. KG expanded their offerings, strengthening their market position. B. Braun SE’s introduction of the SECUREFIX® cranial plate system in September 2023, offering a minimally invasive fixation option, and Medtronic Plc’s launch of the SKYPOINT™ Cranial Fixation System in June 2023, which utilizes 3D-printed titanium for personalized reconstruction, underscore the industry’s advancement. Additionally, Medtronic’s acquisition of Brainlab AG in July 2023 enhances its portfolio with cutting-edge cranial navigation and planning technologies.
Key Takeaways
- Market is projected to hit USD 4 billion by 2033 with a CAGR of 5.6% from 2023’s USD 2.3 billion.
- Cranial Fixation Devices lead, capturing over two-thirds of 2023’s market share, essential for skull stabilization.
- Resorbable Fixation Systems, dissolving over time to avoid second surgeries, held a 56% share in 2023, favored in pediatric cases.
- Hospitals accounted for 62% of the market in 2023, while Ambulatory Surgical Centers are poised for growth.
- Rise in neurological disorders and traumatic brain injuries, particularly among the elderly and accident victims, propels market growth.
- The high costs pose challenges for market expansion, notably in low- and middle-income regions.
- Innovations like biocompatible materials and 3D printing are paving the way for customized implants and improved surgical results.
- Growing preference for Minimally Invasive Surgeries (MIS) lessens hospital stays and infection risks, boosting demand for specialized devices.
- North America led with a 42% market share in 2023, with Europe and Asia-Pacific trailing closely behind.
Emerging Trends
- Customization and Modularity: The trend towards customized and modular cranial fixation and stabilization systems is gaining traction. By tailoring devices to fit individual patient anatomy, these systems can significantly enhance surgical outcomes. This customization allows for more precise fittings of implants and offers surgeons flexible system configurations to better meet specific medical needs. The result is a higher degree of precision in surgeries, potentially improving recovery times and overall patient satisfaction.
- Technological Advancements: Recent technological advancements are revolutionizing cranial fixation devices. Modern devices now feature improved biocompatibility, enhanced strength, and better radiolucency. These improvements facilitate a more comfortable experience for patients and ensure compatibility with advanced imaging technologies. As a result, healthcare providers can achieve a higher level of precision during surgeries, enhancing treatment efficacy and patient safety.
- Integration with Neuro-navigation and Robotics: The integration of neuro-navigation and robotics in cranial surgeries is becoming increasingly prevalent. This trend enhances the precision and accuracy of surgical procedures, which is crucial for optimizing patient outcomes. By utilizing these advanced technologies, surgeons can conduct operations with greater control and accuracy, reducing the risk of complications and improving recovery processes.
- Increased Use of Biocompatible Materials: There is a notable shift towards the use of biocompatible materials in the manufacture of cranial fixation and stabilization devices. These materials are designed to be more compatible with the human body, minimizing the risk of rejection and post-surgical complications. The use of such materials is critical in promoting better patient outcomes and longer-term success of surgical interventions.
- Growth Driven by Neurological Conditions: The increasing prevalence of neurological conditions and traumatic brain injuries (TBIs) is driving demand for advanced cranial stabilization and fixation solutions. As these medical conditions become more common, there is a growing need for innovative solutions that can effectively manage and treat complex cases. This trend underscores the importance of continued innovation and development in the field to keep pace with rising healthcare needs.
Use Cases
- Traumatic Brain Injuries (TBIs): Cranial fixation devices are vital in surgical responses to TBIs. These injuries typically result from accidents or falls. During such emergencies, it’s crucial to stabilize the skull quickly to prevent further damage to the brain. The use of these devices ensures that the affected area remains immobile and protected, which is essential for the patient’s recovery and to minimize long-term neurological damage.
- Neurosurgery Enhancements: In the realm of neurosurgery, precision is not just a requirement but a necessity. Cranial fixation devices play a crucial role here. They secure the patient’s head and prevent any movement that could affect the surgical outcome. This stability is particularly critical during intricate procedures that require exact precision, as even the slightest error can have significant consequences.
- Cranial Reconstruction: For patients who have undergone severe head injuries or surgeries that involve bone removal, cranial fixation devices are indispensable. They help reconstruct the skull, ensuring that the structure of the head is maintained. This support is vital not only during the surgery but also throughout the recovery process, helping the skull to heal correctly and maintain its proper shape.
- Minimally Invasive Surgery: As medical technology advances, surgeries become less invasive, requiring smaller incisions. In these procedures, the stability offered by cranial fixation devices is increasingly important. They ensure that the minimal access points are effectively utilized without compromising the surgical integrity or patient safety, thereby enhancing the overall success of the operation.
- Post-surgical Recovery: After surgery, the role of cranial fixation devices extends into the recovery phase. They are crucial in maintaining the integrity of the cranial structure, which alleviates stress at the surgical site and facilitates healing. This support helps prevent complications and ensures that the recovery process is as smooth and swift as possible, contributing to better outcomes for patients.
Regional Analysis
In 2023, North America dominated the Cranial Fixation and Stabilization Devices Market, securing over 42% of the global market share. The market was valued at USD 0.9 billion, driven by sophisticated healthcare infrastructure, significant healthcare spending, and strong regulatory support for medical device innovation. The United States was crucial in this dominance, thanks to its large population and advanced healthcare system.
Europe also held a notable market share, with Germany, France, and the United Kingdom leading. These countries benefit from well-established healthcare systems, favorable reimbursement policies, and an increasing elderly population. These factors collectively boost the demand for neurosurgical interventions and devices.
Asia-Pacific, though holding a smaller share, saw rapid growth in the market. This surge is attributed to the expanding healthcare infrastructure and growing awareness of neurosurgical procedures, particularly in China, India, and Japan. The region is becoming increasingly significant in the global landscape due to these advancements.
Latin America and the Middle East & Africa experienced steady growth, driven by improving healthcare systems and rising disposable incomes. However, these regions face challenges such as limited healthcare accessibility and regulatory barriers, which temper their market growth potential.
Key Players Analysis
Integra LifeSciences Corporation
Integra LifeSciences Corporation is a key player in the cranial fixation and stabilization devices sector. Their MAYFIELD® product line offers various systems designed to securely position and stabilize the patient’s head during neurosurgical procedures. The MAYFIELD® Standard Cranial Stabilization System provides reliable support, while the MAYFIELD® Imaging Cranial Stabilization System is tailored for imaging applications. Additionally, the MAYFIELD® 2 Skull Clamp enhances precision in head positioning. These devices are essential for ensuring patient safety and surgical accuracy in neurosurgery.
Medtronic Plc
Medtronic Plc is a key player in the cranial fixation and stabilization devices sector. Their INVISx™ Cranial Fixation System is designed to securely reattach the skull flap after craniotomy procedures. This system offers a unique, two-sided clamping mechanism that ensures strong fixation. Additionally, Medtronic’s Stealth Autoguide™ robotic guidance system provides precise stereotactic positioning and trajectory guidance during cranial surgeries, enhancing surgical accuracy. These innovations demonstrate Medtronic’s commitment to advancing neurosurgical procedures and improving patient outcomes.
Depuy Synthes
DePuy Synthes offers a range of products for cranial fixation and stabilization. The MatrixNEURO™ System includes implants with profiles as thin as 0.4 mm, designed to fit various skull shapes, aiming to reduce surgery time. For pediatric and adult patients, the RapidSorb™ Resorbable Fixation System provides a solution that gradually absorbs into the body, eliminating the need for removal surgeries. Additionally, the SYNTHECEL® Dura Repair is used to cover and repair dural defects, preventing cerebrospinal fluid leakage. These offerings highlight DePuy Synthes’ commitment to advancing cranial surgery techniques.
B. Braun SE
B. Braun SE is a key player in the cranial fixation and stabilization devices market. Their CranioFix®2 system, introduced in 1997, has seen over 3.5 million successful implants in more than 60 countries. This titanium clamp system allows for quick and reliable fixation of bone flaps during neurosurgical procedures. Additionally, B. Braun offers CranioFix® absorbable, made from a material that dissolves over 2-3 years, supporting natural skull growth, especially in pediatric patients. These innovations highlight B. Braun’s commitment to advancing neurosurgical care.
Stryker Corporation
Stryker Corporation is a leading medical technology company specializing in cranial fixation and stabilization devices. Their Universal Neuro III system offers a comprehensive selection of low-profile titanium plates, screws, and specialized skull base plates designed for neurosurgical procedures. The system features plates with a low profile height of 0.4mm, deeper countersinks, and smoother geometry to enhance surgical outcomes. Additionally, Stryker’s AXS screws are optimized for improved handling and insertion. These innovations aim to support efficiency and improve surgical outcomes in cranial procedures.
Zimmer Biomet
Zimmer Biomet is a key player in the cranial fixation and stabilization devices sector, offering a comprehensive range of products for neurosurgical procedures. Their 1.5mm Neuro Plating System provides a complete titanium fixation solution, featuring over 25 different plating options and user-friendly instruments, all organized in a self-contained system for convenience. Additionally, the One2One™ HTR-PEKK Patient-Matched Cranial Implant utilizes advanced PEKK material and 3D printing technology to create implants tailored to individual patient anatomy, enhancing surgical outcomes. These innovations underscore Zimmer Biomet’s commitment to advancing neurosurgical care.
Integra Lifesciences
Integra LifeSciences is a key player in the cranial fixation and stabilization devices sector, offering a range of products designed to support neurosurgical procedures. Their MAYFIELD® Standard Cranial Stabilization System provides rigid skeletal fixation, ensuring patient stability during surgery. The MAYFIELD® Imaging Cranial Stabilization System offers nine different configurations to accommodate various imaging requirements. Additionally, the MAYFIELD® Infinity Support System includes multiple rocker arm and torque screw options, enhancing surgical flexibility. These systems are widely used in neurosurgical practices to improve patient outcomes.
KLS Martin
KLS Martin is a prominent company in the cranial fixation and stabilization devices sector, offering a range of products designed to support cranial surgeries. Their L1® Cranium System is utilized for treating fractures, securing bone segments, and reconstructive procedures in the skull area. This system includes ultra-thin plates, measuring 0.35 mm in thickness, which are designed to minimize palpability and integrate seamlessly with the patient’s anatomy. Additionally, KLS Martin provides various distractors and fixators, such as the Cranial Vault Distractor, which is used to treat cranial malformations like syndromic craniosynostosis and congenital deficiencies.
Conclusion
The Cranial Fixation and Stabilization Devices Market is evolving rapidly, driven by technological advancements, increasing demand for minimally invasive procedures, and growing neurological disorders. The adoption of biocompatible materials and 3D printing is enhancing surgical precision and patient recovery. Companies are focusing on innovation, strategic mergers, and product launches to expand their portfolios. North America leads due to strong healthcare infrastructure, while Europe and Asia-Pacific are witnessing significant growth. Despite high costs posing challenges in some regions, the market continues to expand with better accessibility and regulatory support. The rising need for neurosurgical interventions ensures continuous progress, making cranial fixation and stabilization devices essential in modern healthcare.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



