Table of Contents
Introduction
The Companion Diagnostics Market is experiencing robust growth, driven by the confluence of regulatory support and advancements in personalized medicine. Regulatory bodies, notably the FDA, have enhanced the approval processes for drugs that are paired with diagnostics, significantly aiding the development and adoption of these crucial tests. This progress is pivotal for the identification of optimal therapeutic treatments tailored to individual patient profiles, thereby augmenting the efficacy and safety of medical treatments.
Technological advancements play a critical role in this sector’s expansion. Innovations in diagnostic technologies, such as biomarker identification and next-generation sequencing, have markedly improved the precision and reliability of companion diagnostics. These technologies are essential for pinpointing the most beneficial treatments for patients based on their genetic and molecular profiles, which reduces the prevalence of trial-and-error in prescribing practices and elevates health outcomes.
The market is further propelled by strategic collaborations between pharmaceutical and biotech companies. These partnerships are integral for co-developing drugs and their corresponding diagnostics, ensuring that therapeutic products are both effective and timely in their market entry. The growing prevalence of targeted therapies, especially in oncology, underscores the increasing demand for companion diagnostics. These tools are indispensable for determining patient eligibility for specific drugs based on biological markers, thus ensuring effective treatment management.
Recent developments highlight significant strides in the sector. In July 2024, Myriad Genetics introduced the Universal Plus Panel for the Foresight® Carrier Screen, which covers 272 genes linked to serious inherited diseases. This expansion is indicative of the company’s commitment to enhancing diagnostic accuracy and broadening genetic risk assessment capabilities. Similarly, in January 2024, Agilent Technologies teamed up with Incyte to develop sophisticated diagnostics focused on hematology and oncology, aiming to support clinical trials and potential registrations in the U.S. and Europe.
Further reinforcing the market’s dynamics, partnerships like the one between QIAGEN and Myriad Genetics in October 2023 are set to advance the development of companion diagnostics in cancer care. This alliance plans to produce both laboratory-developed tests and kit-based diagnostics for global distribution, utilizing advanced PCR and next-generation sequencing technologies. Such collaborations are pivotal in personalizing cancer treatment based on genetic profiling, marking a critical evolution in the approach to healthcare.
Key Takeaways
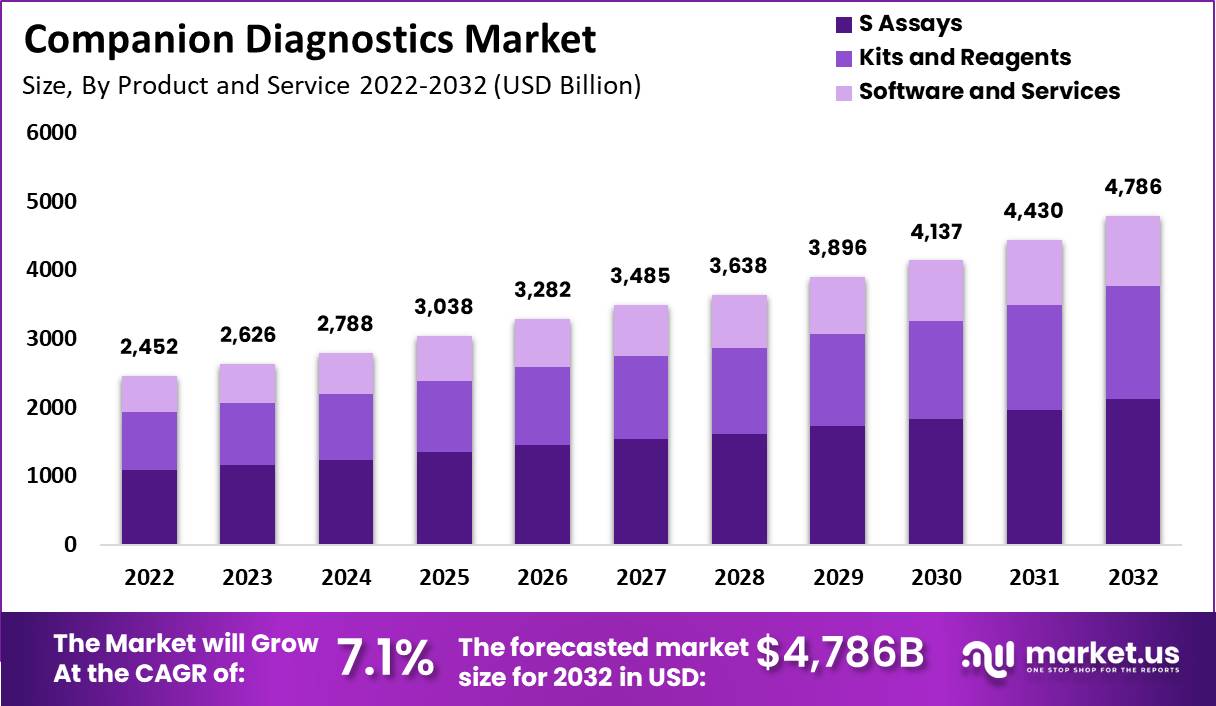
- The global companion diagnostics market is projected to reach $4,786 million by 2032, growing at a 7.1% CAGR, driven by advances in genomics and personalized medicine.
- North America led the market in 2022 with a 43% revenue share, largely due to high cancer rates and advanced healthcare infrastructure.
- Key technologies include polymerase chain reaction (PCR) and next-generation sequencing, with continuous innovation fueling growth.
- Cancer diagnostics dominate, especially in lung, breast, and colorectal cancer, supported by strategic collaborations and FDA approvals.
- Emerging markets and personalized medicine offer significant growth opportunities, despite challenges in reimbursement and regulatory frameworks.
Companion Diagnostics Statistics
- The market size in 2022 was USD 2,452 billion.
- The market size in 2023 is projected to be USD 2,626 billion.
- The market size in 2024 is projected to reach USD 2,788 billion.
- By 2025, the market is expected to grow to USD 3,038 billion.
- The market is projected to reach USD 3,282 billion in 2026.
- By 2027, the market size is expected to grow to USD 3,485 billion.
- In 2028, the market size is projected to reach USD 3,638 billion.
- The market size in 2029 is forecasted to be USD 3,896 billion.
- By 2030, the market size is expected to grow to USD 4,137 billion.
- The market size in 2031 is projected to reach USD 4,430 billion.
- By 2032, the market size is forecasted to grow to USD 4,786 billion.
- The compound annual growth rate (CAGR) of the market from 2022 to 2032 is expected to be 7.1%.
Emerging Trends
- Integration with Smart Devices: Technological advancements have led to the creation of innovative smart diagnostic tools. These devices, including smart toilets and bras, are designed to monitor health metrics continuously. By detecting early signs of conditions like diabetes and breast cancer, these tools offer the possibility of managing health proactively from the comfort of home. This trend highlights the potential for real-time health monitoring and the early detection of diseases, which could revolutionize home healthcare.
- Personalized Healthcare: There is a growing emphasis on precision medicine, which focuses on developing diagnostic tools tailored to individual genetic profiles. This approach seeks to improve treatment efficacy by ensuring that medical treatments are closely aligned with each patient’s unique genetic makeup. Personalized healthcare is rapidly becoming a cornerstone of modern medicine, aiming to optimize treatment outcomes and enhance patient care through tailored diagnostic solutions.
- Expansion of Wearable Diagnostics: Wearable technology is advancing beyond basic health monitoring to include features that predict and prevent diseases. This shift is transforming healthcare from a reactive to a proactive discipline, where continuous monitoring enables the early interception of health issues. The expansion of wearable diagnostics is poised to change how we approach health and disease management, emphasizing prevention and early intervention.
- Increased Research Focus: Historically, diagnostics have received less funding than therapeutic interventions within the healthcare sector. However, there is an increasing recognition of the need to allocate more resources to diagnostic research. Balancing funding between diagnostics and treatments is essential for improving early detection strategies and prevention efforts. This trend acknowledges the critical role of diagnostics in enhancing patient outcomes and overall healthcare efficiency.
Use Cases
- Chronic Disease Management through Wearable Devices: Wearable devices and implantable sensors are revolutionizing the management of chronic diseases such as diabetes and heart conditions. By providing continuous monitoring and collecting real-time data, these technologies enable timely medical interventions. This continuous data flow allows healthcare providers to make personalized adjustments to treatments, ensuring patients receive the most effective care tailored to their specific needs.
- Early Detection of Cancer with Innovative Technologies: Emerging technologies are enhancing the early detection of cancer, potentially increasing treatment success rates. One such innovation includes the development of smart bras, which are equipped with sensors designed to detect breast tumors at an early stage. Early detection is crucial as it significantly boosts the likelihood of successful treatment, offering a substantial advantage in the fight against cancer.
- Enhancing Treatment Efficacy through Genetic Insights: Companion diagnostics are essential in tailoring treatments to individual patients by analyzing their genetic profiles. This personalized approach helps in selecting the most effective treatment options, thereby maximizing therapeutic success and minimizing adverse side effects. The ability to match treatments with patients’ genetic information marks a significant advancement in personalized medicine, making treatments more effective and patient-specific.
- Remote Patient Monitoring Advances: Technologies such as smart toilets and pacemakers are equipped to transmit vital health data remotely to medical professionals. This capability is particularly beneficial for elderly patients or those residing in remote areas, as it reduces the need for frequent hospital visits. Remote monitoring not only provides continuous health surveillance but also ensures that patients receive timely care, enhancing overall healthcare efficiency and patient convenience.
Key Players Analysis
Abbott
Abbott is significantly involved in the companion diagnostics market, a sector crucial for the development of personalized medicine. As a leader in global diagnostics, Abbott focuses on delivering innovative diagnostic solutions that enhance clinical decision-making and improve patient outcomes. Their work in the companion diagnostics space particularly supports therapies that require precise patient stratification and treatment optimization. This strategic focus is evidenced by their ongoing investment in next-generation systems, aimed at driving growth in this sector through improved accuracy and operational efficiencies in healthcare settings.
Agilent Technologies Inc.
Agilent Technologies Inc. has recently enhanced its position in the companion diagnostics (CDx) sector through a strategic collaboration with Incyte. This partnership focuses on developing advanced CDx solutions, particularly for hematology and oncology applications. This initiative is part of a broader effort to tailor medical treatments more effectively, especially in cancer care, by leveraging biomarkers to match patients with the most suitable therapeutic options. This collaboration aims to support clinical trials and the potential registration and commercialization of these diagnostics in key markets such as the United States and Europe.
F. Hoffmann-La Roche Ltd. (Switzerland)
F. Hoffmann-La Roche Ltd has made significant strides in the companion diagnostics (CDx) sector, particularly with a recent partnership in February 2024 with PathAI. This collaboration aims to develop AI-enabled pathology algorithms, enhancing their CDx portfolio through advanced technological integration. Roche’s commitment to improving precision in cancer treatment is further evidenced by their broad involvement in diagnostic tests across multiple cancer types, including lung, breast, and colorectal cancers.
Guardant Health
Guardant Health has significantly advanced in the field of companion diagnostics within the oncology sector, especially through the use of its Guardant360® CDx liquid biopsy test. This test has received multiple FDA approvals for its use in identifying specific mutations in patients with advanced or metastatic cancers, such as breast cancer and non-small cell lung cancer (NSCLC), to guide targeted therapy options. For instance, Guardant360® CDx has been approved as a companion diagnostic for treatments targeting ESR1 mutations in breast cancer and for detecting EGFR exon 20 insertion mutations in NSCLC patients.
The test offers a robust alternative to traditional tissue biopsies by providing comprehensive genomic profiling from a simple blood draw, delivering results within a week. This rapid turnaround is crucial for timely and personalized treatment decisions. The widespread adoption of this test is reflected in its extensive clinical use, trusted by over 12,000 oncologists and covering over 230 million lives through various health plans. This acceptance and validation in the medical community underscore its pivotal role in enhancing patient outcomes by enabling more precise and personalized cancer treatment strategies.
QIAGEN (Germany)
QIAGEN has been actively developing its presence in the companion diagnostics sector, particularly in oncology, where it leverages advanced molecular technologies like PCR and next-generation sequencing (NGS). Recently, QIAGEN expanded its partnerships, notably with Myriad Genetics, to enhance the development and commercialization of companion diagnostic tests aimed at improving cancer treatment. This collaboration focuses on integrating Myriad’s clinical testing capabilities with QIAGEN’s robust technological platforms and global commercial channels, providing comprehensive solutions to pharmaceutical partners worldwide.
Furthermore, QIAGEN’s approval by the FDA for its therascreen PDGFRA RGQ PCR kit marks a significant milestone. This kit aids in identifying patients with gastrointestinal stromal tumors suitable for treatment with specific therapeutics, highlighting QIAGEN’s commitment to advancing precision medicine through innovative diagnostic solutions.
These developments underscore QIAGEN’s strategic focus on expanding the utility of companion diagnostics across various therapeutic areas, including hereditary diseases and complex conditions like autoimmune disorders and central nervous system diseases. The company’s extensive global network and regulatory expertise facilitate the swift commercialization of these diagnostics, ensuring they are accessible for clinical use at the outset of new drug launches.
Myriad Genetics, Inc.
Myriad Genetics, Inc. is significantly enhancing the field of companion diagnostics, especially within oncology. A key aspect of their work is their collaboration with QIAGEN, aiming to advance and globalize cancer diagnostics. This partnership focuses on developing assays that integrate next-generation sequencing and digital PCR technologies, enhancing the precision and accessibility of cancer treatment options globally.
Recently, Myriad received FDA approval for their BRACAnalysis® CDx test for use in identifying patients with certain types of early-stage breast cancer, which is pivotal in guiding treatment options with PARP inhibitors, a method shown to improve survival rates significantly. Additionally, their myChoice CDx® test has been approved for advanced ovarian cancer treatments, supporting targeted therapies that improve patient outcomes.
These advances underscore Myriad’s commitment to integrating cutting-edge science to improve diagnostic accuracy and treatment efficacy, reflecting their broader strategy to enhance patient care through precision medicine and tailored treatments.
Illumina Inc.
Illumina Inc. is actively involved in advancing the field of companion diagnostics, particularly within oncology, leveraging its genomic sequencing capabilities to enhance precision medicine. A significant development is Illumina’s partnership with pharmaceutical companies like Merck to develop tests that determine the eligibility of patients for specific cancer treatments based on genetic mutations. This collaboration focuses on leveraging the TruSight™ Oncology 500, which provides comprehensive genomic profiling from a single test, helping to identify actionable cancer mutations for targeted therapies.
Recently, Illumina introduced a new pan-cancer companion diagnostic tool as part of its TruSight™ Oncology Comprehensive test in Europe. This tool enables the identification of patients with rare genetic mutations who might benefit from targeted therapies like Bayer’s VITRAKVI® for NTRK fusion cancer, demonstrating a forward step in multi-tumor precision oncology.
These efforts underline Illumina’s strategic direction towards expanding the utility and application of genomic profiling in therapeutic decision-making across various cancers, enhancing treatment efficacy and personalization
Thermo Fisher Scientific Inc.
Thermo Fisher Scientific Inc. is making significant strides in the companion diagnostics sector, primarily through its Thermo Fisher Center for Multiplexed Proteomics (TCMP) at Harvard Medical School. This center, operational since 2014, focuses on multiplex quantitative proteomic expression profiling, a crucial aspect of developing companion diagnostics which are essential for personalizing patient care by matching therapeutic products to the biological markers defined through the diagnostics process. Utilizing advanced technologies like high-resolution mass spectrometers and isobaric tandem mass tags, the TCMP can analyze multiple samples concurrently, enhancing the precision and efficiency of protein or phosphopeptide expression profiling. This capability directly supports the development of more effective and targeted therapeutic strategies, solidifying Thermo Fisher’s role in advancing companion diagnostics.
BIOMERIEUX (France)
BioMérieux, a key player in the field of in vitro diagnostics, has demonstrated a robust presence in the companion diagnostics sector. In 2023, the company reported significant growth, with annual sales reaching approximately €3.675 billion, marking a 6.6% organic growth. This performance underscores the company’s effective response to market demands, particularly in non-respiratory diagnostic panels and microbiology, where they observed a notable sales increase. bioMérieux’s strategic focus also includes expanding its product portfolio in companion diagnostics, emphasizing solutions that support precise therapies and enhance patient outcomes. Their ongoing initiatives to innovate and adapt to the evolving healthcare environment signify their commitment to maintaining a significant role in the companion diagnostics market.
Myriad Genetics Inc.
Myriad Genetics, Inc. is a prominent player in the companion diagnostics sector, focusing on genetic testing and precision medicine. The company collaborates with pharmaceutical giants like AstraZeneca and Merck, particularly in using their BRACAnalysis CDx® test. This test identifies patients with BRCA mutations, helping tailor targeted therapies for various cancers, including ovarian, breast, and prostate cancer. In 2023, Myriad also partnered with QIAGEN to expand their offerings, developing cancer diagnostic tests that support personalized treatment decisions globally. This collaboration is expected to enhance Myriad’s capabilities, making companion diagnostics more accessible and effective in guiding cancer treatment decisions.
Conclusion
The Companion Diagnostics Market is expanding rapidly due to technological advancements, growing personalized medicine adoption, and strong regulatory support. Strategic collaborations between pharmaceutical and biotech companies are enhancing the development of precise diagnostic tools. These partnerships are crucial for co-developing drugs and companion diagnostics, particularly in cancer treatment, ensuring timely and effective market entry. Despite challenges like reimbursement issues and regulatory complexities, the market is poised for sustained growth. The increasing focus on tailored treatments and the expansion of diagnostic technologies, such as next-generation sequencing, underline the market’s potential. This evolution is essential for improving patient outcomes and driving the future of personalized healthcare.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



