Introduction
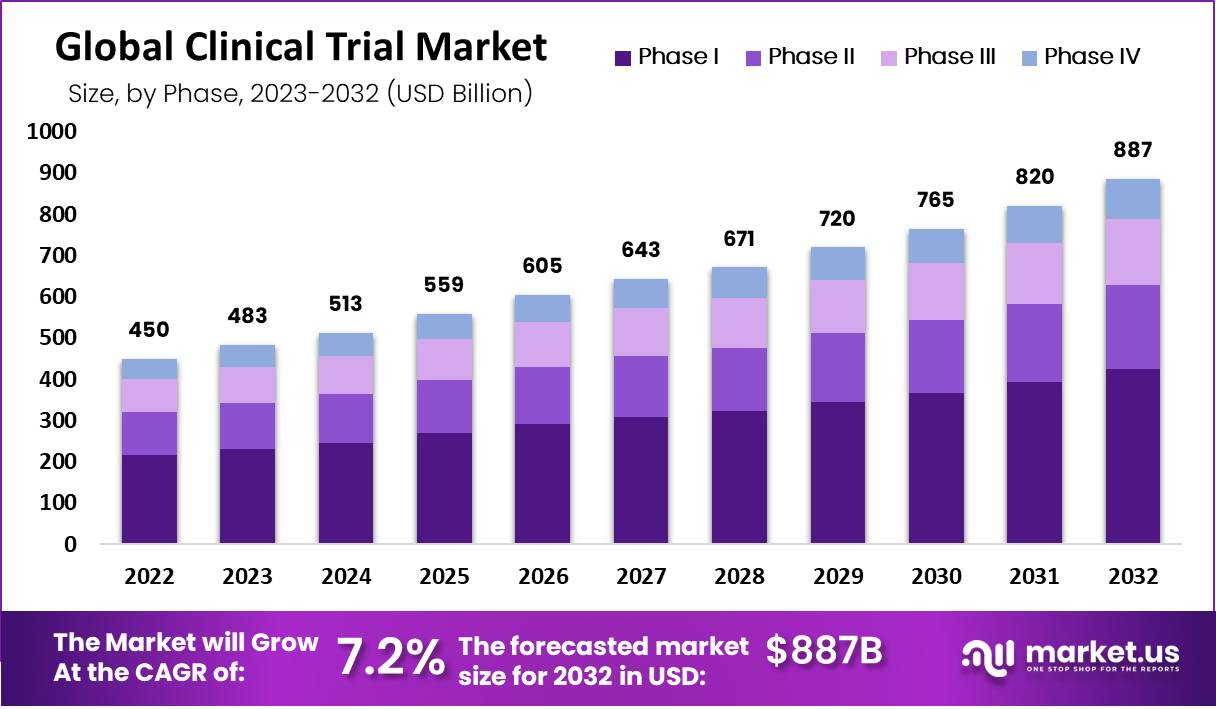
Global Clinical Trials Market size is expected to be worth around US$ 886.5 Billion by 2032 from US$ 483 Billion in 2023, growing at a CAGR of 7.2% during the forecast period from 2023 to 2032. In 2023, North America led the market, achieving over 47.2% share with a revenue of US$ 212.4 Billion.
This growth is primarily driven by the increasing demand for personalized medicine, which focuses on tailoring treatments to individual genetic profiles. These personalized approaches improve therapy efficacy across diverse populations, necessitating more nuanced and comprehensive clinical trials.
Technological advancements are playing a pivotal role in enhancing the efficiency of clinical trials. Innovations such as artificial intelligence (AI) and machine learning are streamlining data analysis, accelerating the drug development and approval process. Moreover, regulatory agencies are providing support through streamlined procedures and funding, particularly for research targeting rare diseases and urgent public health challenges.
The rising prevalence of chronic diseases is another key driver of market growth. Conditions like cancer and cardiovascular diseases have increased the demand for new, effective treatments, leading to a surge in clinical trials targeting these ailments. The expansion of Clinical Research Organizations (CROs) has also contributed by enabling trials across diverse genetic and cultural populations, supporting the global reach of pharmaceutical innovations.
Recent market developments highlight its dynamic and evolving nature. For instance, Charles River Laboratories’ acquisition of Vigene Biosciences has strengthened its gene therapy manufacturing capabilities, showcasing the industry’s focus on advanced therapeutics. Additionally, Parexel International’s privatization by Pamplona Capital Management is expected to enhance its operational flexibility and ability to capitalize on market opportunities.
AI adoption is transforming the clinical trials sector, optimizing drug discovery and trial management. Regulatory efforts, particularly by the FDA, are promoting greater trial diversity, ensuring more inclusive and effective treatment development. Geographically, while North America dominates the market, the Asia-Pacific region is witnessing rapid growth due to government initiatives and a rising demand for healthcare solutions, particularly for chronic diseases.
The clinical trials market continues to evolve, driven by technological advancements, regulatory changes, and the growing need for personalized and inclusive healthcare solutions worldwide. This progression signals a promising future for global health innovation.
Key Takeaways
- The Clinical Trials Market is projected to reach US$ 886.5 billion by 2032, growing at a CAGR of 7.2% from 2022.
- As of 2022, the market valuation stood at US$ 450.1 billion.
- The oncology segment remains the largest revenue contributor, while cardiovascular studies are expected to increase.
- Pharmaceutical and biopharmaceutical companies hold a dominant market share.
- With an uptick in clinical trials, CROs are witnessing significant growth.
- Outsourcing clinical trials has become a notable trend that supports market growth.
- North America leads in market share due to robust technological innovations and R&D investments.
- The Asia-Pacific region is witnessing rapid growth, fueled by the pandemic’s impact and a large patient base.
- Key market players include Eli Lilly, Parexel, Pfizer, and IQVIA, leading innovations and expansions.
Indication Analysis
- Pain Management: Clinical trials in pain management are investigating a range of treatments, including novel analgesic drugs and non-pharmacological interventions. For instance, trials are exploring the effectiveness of scrambler therapy versus transcutaneous electrical nerve stimulation (TENS) for chemotherapy-induced peripheral neuropathy, which involves nerve pain.
- Oncology: Oncology remains a heavily researched area in clinical trials, focusing on new drug developments, combination therapies, and personalized medicine approaches. These trials aim to improve outcomes in various cancers through targeted therapies and innovative treatment protocols.
- CNS Conditions: Clinical trials targeting CNS conditions often investigate new drugs or novel therapeutic approaches for diseases such as multiple sclerosis, Parkinson’s disease, and Alzheimer’s. The trials may include interventions aiming to modify disease progression or alleviate symptoms.
- Diabetes: In diabetes, clinical trials frequently focus on new therapeutic agents, management strategies, and technologies aimed at improving glycemic control and reducing complications. These studies might involve new insulins, oral medications, or devices like continuous glucose monitors.
- Obesity: Trials in obesity are increasingly important due to the rising prevalence globally. These studies test interventions ranging from pharmacological treatments, dietary supplements, to lifestyle modification programs, aiming to understand their efficacy in weight management and related metabolic conditions.
Emerging trends
- Integration of Real-World Evidence (RWE): Regulatory bodies like the FDA and EMA are increasingly advocating for the inclusion of Real-World Evidence in clinical trials to expedite the approval of new treatments. RWE enhances clinical trial designs by providing data that reflect real-world patient outcomes, thus supporting better decision-making and accelerating the availability of innovative therapies.
- Advancements in Biomarker Identification: The identification of new biomarkers, particularly those derived from wearable technologies, is revolutionizing clinical trials. These biomarkers allow for the continuous monitoring of patients’ physiological and behavioral data, facilitating the development of personalized treatments tailored to individual patient profiles.
- Rise of Decentralized Clinical Trials (DCTs): DCTs are gaining popularity as they improve patient accessibility and engagement by leveraging digital platforms to collect data remotely. This approach minimizes logistical barriers, enhances patient participation, and increases the efficiency and inclusivity of clinical research.
- Focus on Inclusion in Emerging Markets: There’s a growing emphasis on including diverse and underrepresented populations in clinical trials, driven by a global initiative towards health equity. This ensures that health advancements are accessible and beneficial to all demographic groups, addressing a wider spectrum of medical needs.
- Enhancement of Data Visualization Tools: With the increasing complexity of clinical data, sophisticated data visualization tools are becoming essential. These tools help in the accurate interpretation and presentation of data, ensuring compliance with regulatory standards and facilitating clear communication of research findings.
- Modernization of Clinical Trial Platforms: Platforms like ClinicalTrials.gov are being modernized to enhance user experience and improve trial management. These upgrades make trial information more accessible and streamline the interface for researchers and participants, fostering a more efficient research environment.
Use Cases
- Efficient Trial Design: By integrating RWE, clinical trials can more accurately reflect real patient demographics, which streamlines trial design and speeds up the introduction of new therapies to the market.
- Patient Stratification: Advanced biomarkers are used for patient stratification, improving the precision of clinical interventions and personalizing treatment approaches. This leads to better clinical outcomes and enhanced treatment efficacy.
- Remote Patient Monitoring: DCTs utilize technology to monitor patients remotely, expanding the reach of trials and increasing the diversity of participant pools. This contributes to more comprehensive and reliable trial results.
- Global Health Initiatives: Modern trials focus on inclusivity and representation, aiding global health initiatives by addressing health disparities and enhancing health outcomes across different regions.
- Regulatory Compliance and Reporting: Advanced data visualization tools improve the management and interpretation of complex data, ensuring adherence to regulatory standards and supporting quicker review and approval processes.
Key Player Analysis
Eli Lilly and Company: A significant participant in clinical trials, focusing on areas like oncology, metabolic disorders, and neurological diseases. Eli Lilly is noted for its extensive trials with Verzenio, a cancer treatment drug, reflecting its robust commitment to developing advanced medical therapies.
Parexel International Corporation: Known for its extensive range of clinical development services from Phase I to IV, Parexel integrates advanced technologies, including artificial intelligence, to enhance trial outcomes. The company has also made commitments to environmental sustainability, setting science-based targets for reducing greenhouse gas emissions.
Pfizer: Actively engaged in enhancing the clinical trials landscape, especially in oncology and chronic diseases. Pfizer is advancing significant trials in 2024, such as those for atirmociclib, a new breast cancer treatment, while focusing on improving patient experience and engagement in clinical trials.
Charles River Laboratories: Provides comprehensive laboratory services that support drug development. Charles River focuses on early-stage integration to help accelerate clinical timelines, offering services from bioanalysis to clinical kitting. The company has also expanded its expertise into gene therapy, supporting trials for complex diseases.
Syneos Health: Specializes in contract research with a broad service offering that spans clinical development to commercialization. Syneos Health has engaged in strategic partnerships to enhance decentralized clinical trials (DCTs), improving trial efficiency and patient access through technology.
Novo Nordisk A/S: Continues to drive progress in diabetes and obesity treatments. In 2023, Novo Nordisk reported substantial growth driven by its diabetes and obesity care products, underscoring its commitment to addressing these significant health issues with innovative clinical trials.
IQVIA: Focuses on data-driven and technology-enhanced trial methodologies to improve trial performance. IQVIA’s 2024 report noted a rise in clinical development productivity, attributed to innovative trial designs and the use of digital and decentralized approaches.
ICON Plc.: Stands out for its expertise in healthcare intelligence and clinical research, showing strong financial health and receiving industry accolades for clinical research excellence and technological innovation in trials.
Conclusion
The clinical trials market is poised for significant growth, driven by the demand for personalized medicine, technological advancements, and the increasing prevalence of chronic diseases. As the sector evolves, key trends such as the integration of Real-World Evidence, advancements in biomarker identification, and the rise of decentralized clinical trials are shaping the future of drug development.
With a focus on inclusivity and improved efficiency, these innovations promise to enhance global health outcomes. Prominent market players like Eli Lilly, Pfizer, and IQVIA are at the forefront, leveraging new technologies and methodologies to optimize trial efficacy and accelerate the delivery of healthcare solutions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



