Overview
New York, NY – Aug 22, 2025 – In 2023, the Global Clinical Trial Imaging Market was valued at USD 1,150.5 Million. Between 2025 and 2032, this market is estimated to register the highest CAGR of 7.8%. It is predicted to reach USD 2,219.8 Million by 2032.
The clinical trial imaging market is witnessing robust growth, driven by the increasing adoption of imaging modalities to enhance drug development and regulatory compliance. Imaging techniques, including MRI, CT, ultrasound, and PET, are being widely deployed to generate reliable and quantifiable endpoints that accelerate clinical trials and improve patient outcomes.
The expansion of precision medicine and targeted therapies has created strong demand for imaging solutions that provide deeper insights into disease progression and therapeutic efficacy. Clinical trial imaging is increasingly being integrated into oncology, neurology, and cardiovascular studies, where accurate visualization plays a crucial role in validating trial data. Furthermore, the rising prevalence of chronic diseases and the growth of global clinical research activities are expected to support market expansion.
Technology advancements, such as artificial intelligence (AI)-driven image analysis, are enhancing efficiency by reducing variability and improving accuracy. These innovations enable faster decision-making and cost savings for pharmaceutical and biotechnology companies. Additionally, partnerships between imaging service providers, contract research organizations, and drug developers are strengthening the market ecosystem.
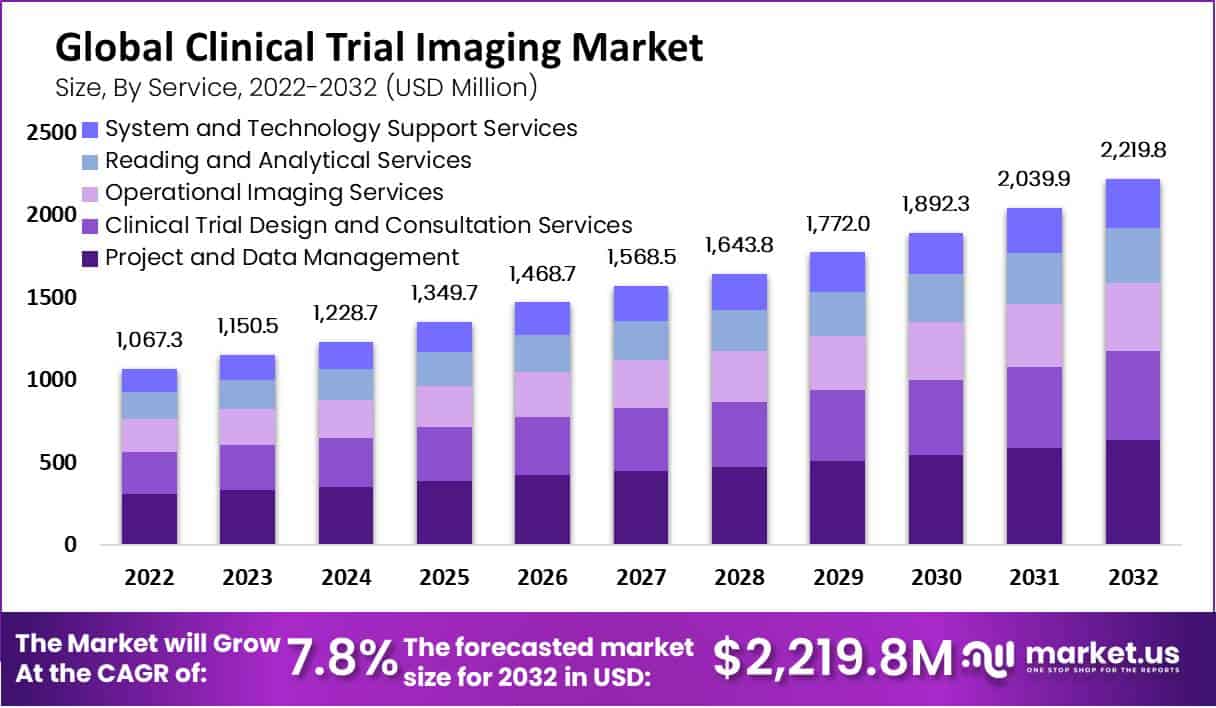
The clinical trial imaging market is projected to achieve steady growth, supported by rising R&D investments, regulatory emphasis on imaging biomarkers, and increasing global trial volumes. This progress reflects the industry’s commitment to delivering safe, effective, and innovative therapies to patients worldwide.
Key Takeaways
- Regional Insights: North America accounts for the largest revenue share, representing 39.6% of the global clinical trial imaging market. The Asia Pacific region is projected to expand at a rapid pace, supported by a growing patient base, lower clinical study costs, and strengthening regulatory frameworks.
- End-User Dynamics: Contract Research Organizations (CROs) remain the leading end-users, contributing 45.8% of market revenue, followed by biotechnology and pharmaceutical companies.
- Market Restraints: Growth is hindered by the high radiation risks associated with medical imaging devices and the expensive nature of these technologies.
- Service Landscape: Project and data management services dominate the service segment, accounting for 28.6% of the global share.
- Quality Standards: The adoption of new protocols, such as QIBA, is improving the accuracy, consistency, and reliability of imaging in clinical trials.
- Opportunities: Rising healthcare expenditure across multiple countries is expected to create significant opportunities for market expansion.
- Competitive Landscape: Key players operating in the market include Keosys, Navitas Life Sciences, Radiant Sage LLC, Resonance Health, Medpace, Biomedical Systems Corp, WCG Clinical, BioTelemetry, IXICO plc, and Icon PLC.
Regional Analysis
The global clinical trial imaging market is led by North America, which accounts for the largest revenue share of 39.6%. This dominance is primarily driven by the high prevalence of chronic diseases and the rising proportion of the aging population. Increasing healthcare expenditure by governments in the United States and Canada over recent years has further strengthened the region’s position. In addition, the strong presence of pharmaceutical companies and their growing investment in research and development (R&D) activities have significantly contributed to market growth in North America.
Following North America, the Asia Pacific region is projected to experience rapid growth during the forecast period. Key factors supporting this expansion include a large and expanding patient population, lower costs associated with clinical studies, and favorable regulatory environments. These dynamics are making Asia Pacific an increasingly attractive hub for clinical trial imaging, driving substantial opportunities for market participants.
Clinical Trial Imaging Market FAQ’S
- What is the current size of the clinical trial imaging market?
The clinical trial imaging market was valued at USD 1,150.5 Million in 2023 and is projected to grow at a CAGR of 7.8%, driven by the increasing number of drug development pipelines and the rising adoption of imaging biomarkers. - What factors are driving growth in this market?
Growth is driven by the rise in chronic diseases, demand for advanced imaging biomarkers, outsourcing of imaging services, and technological advancements in image analysis powered by artificial intelligence (AI). - Who are the major players in the clinical trial imaging market?
Leading players include ICON plc, Parexel, BioTelemetry (Philips), Radiant Sage, Bioclinica (Clario), and Medpace, among others. These companies provide centralized imaging solutions and end-to-end trial support. - What are the main service types in the clinical trial imaging market?
The market is segmented into project management, image acquisition, image analysis, image data management, and consulting services. Image analysis and data management hold the largest share. - Which regions dominate the market?
North America holds the largest share due to advanced infrastructure and regulatory frameworks, while Asia-Pacific is expected to register the fastest growth owing to increasing clinical trial outsourcing and expanding pharmaceutical activity. - What role does AI play in the clinical trial imaging market?
AI enhances image analysis by enabling automated detection, faster interpretation, and improved accuracy. It also helps reduce time and cost in trials while increasing reproducibility of imaging endpoints. - What are the restraints to market growth?
Key restraints include high operational costs, shortage of skilled radiologists, complex regulatory requirements, and challenges in data harmonization across global trials.
Conclusion
The clinical trial imaging market is poised for sustained expansion, underpinned by rising chronic disease prevalence, increasing global clinical research activities, and the adoption of advanced imaging modalities as critical tools for drug development. North America dominates the market, while Asia Pacific is emerging as a high-growth region due to cost advantages and favorable regulations.
Technological advancements, particularly AI-driven image analysis, are improving accuracy, efficiency, and trial outcomes. Despite challenges such as high costs and radiation risks, growing R&D investments, stronger regulatory emphasis on imaging biomarkers, and expanding partnerships across the ecosystem will continue to drive market growth.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



