Table of Contents
Overview
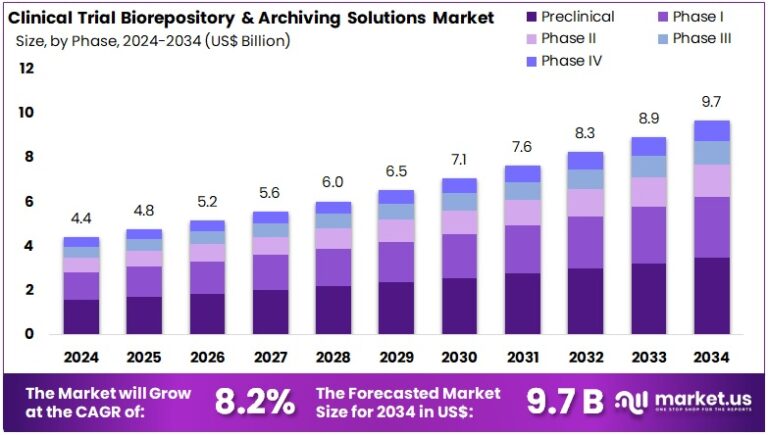
New York, NY – Aug 05, 2025 : The Global Clinical Trial Biorepository and Archiving Solutions Market is projected to grow from US$ 4.4 Billion in 2024 to US$ 9.7 Billion by 2034, at a CAGR of 8.2%. North America leads with a dominant 47.2% share, valued at US$ 2.0 Billion. This growth is fueled by increasing global clinical research activity. As trials grow in scale and complexity, the demand for secure biospecimen storage rises. Efficient biorepositories are now essential to ensure sample integrity and long-term value for research purposes.
Technological advancements have revolutionized biospecimen management. Automated storage and digital tracking tools have improved accuracy and data quality. These solutions support better sample tracking, timely retrieval, and long-term viability. For example, Phase III trials may generate over 3.6 million data points. Such scale demands reliable and scalable biobanking infrastructure. Researchers now rely on these technologies for fast and informed decision-making during trials. Modern systems ensure precision, reduce human error, and support the growing complexity of global clinical trials.
Standardization and data consistency are now core priorities. Uniform sample processing and quality control measures help maintain consistency across research sites. Initiatives like the CDC’s National ALS Biorepository link biological and epidemiological data. This promotes reliable, high-quality data collection for neurodegenerative disease research. Global efforts are focused on improving transparency and reproducibility. These measures help ensure sample traceability and optimize their use in long-term research. Consistent procedures are also vital for cross-border studies and data-sharing collaborations.
Regulatory guidelines support the expansion of biorepositories. Organizations like the WHO and IARC promote structured biobanking frameworks. The IARC biobank holds over 6 million samples for gene environment interaction studies. These initiatives benefit researchers in low- and middle-income countries. They enable equitable access to high-quality biospecimens and research support. Regulatory backing ensures ethical practices and encourages broader participation in biomedical research. Such support also facilitates international collaboration and enhances the overall reliability of clinical trials.
Personalized medicine is driving the need for well-structured biorepositories. Tailored treatments rely on diverse biological data to identify precise biomarkers. Biobanks store highly characterized samples that support translational research. However, gender diversity in trials remains a concern. From 2019 to 2023, male-only trials in the UK were 67% more common than female-only ones. Pregnant and breastfeeding women were often excluded. This highlights the need for inclusive data collection and accessible biospecimen storage. Biorepositories play a critical role in supporting personalized and inclusive healthcare innovations.
Key Takeaways
- A recent report reveals the Clinical Trial Biorepository and Archiving Solutions Market is set to grow from US$ 4.4B in 2024 to US$ 9.7B by 2034.
- Analysts predict the market will expand at a consistent CAGR of 8.2% between 2025 and 2034, driven by rising global trial volumes.
- Biorepository Services led the service segment in 2024, claiming over 66.7% of market share due to increasing demand for long-term sample storage.
- Clinical Products dominated the product category in 2024, securing a 48.4% share thanks to demand for compliant, standardized storage containers and supplies.
- The Preclinical phase accounted for more than 37.2% of market share in 2024, reflecting a strong emphasis on early-stage clinical research.
- North America held the largest regional share at over 47.2% in 2024, with the market reaching a value of US$ 2.0 billion.
Regional Analysis
In 2024, North America held a dominant position in the Clinical Trial Biorepository and Archiving Solutions Market. The region captured more than a 47.2% share, with a market value of US$ 2.0 billion. This leadership is mainly due to the high volume of ongoing clinical trials. The United States contributes significantly, thanks to its advanced healthcare infrastructure and strong regulatory system. The presence of major pharmaceutical and biotech firms has increased the demand for efficient biorepository and archiving services across the region.
Strict regulatory compliance, led by authorities such as the FDA, has driven the adoption of digital archiving systems. These solutions help research institutions manage data securely and ensure traceability throughout clinical trials. Additionally, the rise of personalized medicine and genetic research is fueling demand for advanced sample storage systems. Public and private investments are boosting this growth by enhancing biorepository infrastructure. Due to innovation, regulation, and funding, North America is expected to maintain its market leadership through the forecast period.
Segmentation Analysis
Service Segment Analysis
In 2024, Biorepository Services led the service segment of the Clinical Trial Biorepository and Archiving Solutions Market with over a 66.7% share. This growth was fueled by the increasing need for secure storage of biological samples. Samples like blood, tissue, plasma, and DNA must be stored under strict conditions to ensure integrity. The rise of personalized medicine and genomics has also driven demand. Regulatory bodies like the FDA and EMA require long-term sample retention. Healthcare firms are investing heavily in compliant, standardized storage systems.
Product Segment Analysis
The Clinical Products segment held the largest share in 2024, capturing more than 48.4% of the product market. This is due to the growing number of clinical trials globally. These trials generate large volumes of samples, such as serum and plasma. Strict traceability requirements have made reliable storage essential. The Preclinical Products segment is also expanding. This includes toxicology and early drug development samples. Other fast-growing categories include human tissue, organs, and stem cells. These are crucial for regenerative and genetic research, especially in niche medical areas.
Phase Segment Analysis
Preclinical trials held a dominant 37.2% market share in the phase segment in 2024. These trials need strict biospecimen handling and archiving systems for toxicology and genetic analysis. Phase I followed closely, driven by growing demand for secure storage in safety trials. Proper archiving is vital for compliance and data integrity. Phases II and III contributed steadily, needing large-scale facilities for sample storage and data tracking. Phase IV saw slower growth but is gaining importance. It supports post-marketing studies and long-term patient safety evaluations.
Key Players Analysis
The Clinical Trial Biorepository and Archiving Solutions Market is driven by key players ensuring quality, compliance, and operational efficiency. Companies such as Medpace and ATCC lead with strong biosample storage and documentation solutions. Medpace provides integrated services for both ambient and cryogenic conditions, aligning with global trial needs. ATCC offers a vast, authenticated biological material repository and strict quality standards. These capabilities enhance data traceability, reproducibility, and support Good Clinical Practice (GCP) compliance across clinical studies conducted in multiple regions worldwide.
Cell&Co BioServices and Brooks Life Sciences are also key contributors to this market. Cell&Co delivers ISO-compliant solutions and ethical sample handling for sponsors and CROs, with a focus on Europe. Brooks Life Sciences, now part of Azenta Life Sciences, supports large-scale, temperature-sensitive trials using automated storage and advanced cold chain systems. Thermo Fisher Scientific Inc. further strengthens the market with broad biobanking services and global regulatory support. Academic centers, CROs, and regional biorepositories add diversity, especially in emerging markets, through localized services and infrastructure expansion.
Emerging Trends
1. Personalized Medicine is Driving Biorepository Growth
The rise of personalized medicine is changing clinical research. More trials now focus on cancer, rare diseases, and gene-based therapies. These studies require collecting and storing a large number of biological samples like blood, DNA, and tissue. Biorepositories are adapting to this shift. They’re improving their storage methods and labeling systems. This ensures that every sample remains identifiable and intact. Proper tracking also supports future research use. As personalized treatments grow, so does the demand for advanced, scalable, and compliant biorepository solutions across clinical trial settings.
2. Digital Archiving and Automation Are Replacing Paper
Clinical trial documentation is moving away from paper. More companies are switching to digital archiving platforms. These systems use cloud storage and real-time updates for better accuracy. Automation tools are also becoming popular. They help manage samples, reduce manual tasks, and lower the chances of errors. These digital solutions make it easier to store, retrieve, and audit data. Overall, digitization improves transparency and compliance. It also saves time, especially in global clinical trial environments.
3. Rising Regulatory Pressure on Storage and Retention
Governments and health agencies are asking clinical trial sponsors to store samples and records for longer periods. New regulations demand better data retention, traceability, and safety. As a result, companies are investing in secure and compliant archiving solutions. These include high-capacity storage, digital logs, and audit trails. The focus is on long-term preservation without compromising sample quality. Strong regulatory frameworks are pushing the industry to standardize processes. This ensures proper documentation and sample integrity throughout the trial lifecycle.
4. Decentralized Trials Increase Need for Regional Storage
Clinical trials are becoming more global and decentralized. Many are now conducted across multiple countries and even in home-care settings. This creates challenges for sample storage and transport. In response, companies are building regional storage hubs. These facilities help reduce transit times and maintain sample conditions. Courier-integrated logistics are also being used. They ensure safe, temperature-controlled delivery from collection sites to labs. This trend supports faster data collection and improves trial efficiency worldwide.
5. Cold Chain Logistics Are Now Essential
Cold chain management is critical in clinical trials. Many samples must be kept cold or frozen to remain viable. Biorepositories are now equipped with advanced refrigeration and cryopreservation systems. These facilities use real-time monitoring to track temperature changes. They also include backup systems to prevent sample damage during power outages. This level of care ensures high-quality samples for research. As trials become more complex, cold chain solutions are vital for compliance and data accuracy.
6. Blockchain Use Is Growing for Sample Traceability
Blockchain is starting to enter the clinical trial space. It offers a secure and transparent way to track biological samples. Each transaction is recorded and cannot be changed. This builds trust and ensures compliance with strict regulations. While adoption is still early, some biorepositories are testing blockchain for sample tracking and auditing. It can also help prevent fraud and errors. As the technology matures, it may become a standard tool for improving traceability and data integrity in the industry.
Use Cases
1. Sample Storage for Long-Term Drug Research
Pharmaceutical companies store biological samples from clinical trials for several years. These samples may include blood, tissue, or DNA. When new side effects are discovered later, researchers can test the old samples again. This avoids starting from scratch. Stored samples help scientists check how patients responded over time. They also help in discovering new uses for existing drugs. Long-term storage supports both drug safety and innovation. Proper biorepository services ensure that samples remain stable and usable. This is critical for accurate future testing. It also saves time and costs for pharmaceutical firms.
2. Regulatory Audits and Trial Documentation
Clinical trials must follow strict rules set by global authorities. During audits, regulators may ask for past records and documents. These can include patient consent forms, trial reports, and protocols. Archiving solutions keep all of this data secure and organized. This makes it easier to retrieve documents, even many years later. Fast access to accurate records builds trust and avoids legal issues. Proper archiving also supports transparency in research. Biorepository systems often include digital storage for easy search and retrieval. This helps companies stay ready for any regulatory inspection.
3. Cross-Trial Data Comparison
Sometimes, researchers need to compare results from different clinical trials. This helps identify patterns or improve how new studies are designed. Well-managed biorepositories allow quick access to old trial data and samples. Organized storage makes cross-trial analysis easier and more accurate. Researchers can use this data to validate new findings. It also helps confirm drug effectiveness across different groups. These comparisons often lead to better treatment plans. Without proper storage, valuable data could be lost. Biorepositories ensure researchers get the right data at the right time.
4. Supporting Rare Disease Research
Rare diseases affect very few people, so collecting samples is hard. Biorepositories help by storing rare disease samples for future use. This makes it easier for scientists to study rare conditions over time. They don’t have to wait to find new patients. Stored samples are often used to test new treatments or validate early results. Researchers also use them to understand how rare diseases develop. Proper storage ensures that the samples stay in good condition. This helps in generating accurate research outcomes and speeds up drug development.
5. Enhancing AI and Machine Learning Models
Artificial intelligence is becoming more common in clinical research. These tools need high-quality data to learn and improve. Biorepositories store both biological samples and related trial data. This data is used to train AI models to predict patient outcomes or drug responses. It helps in identifying which treatments work best for different groups. Stored samples also allow scientists to test AI-generated insights. Proper data archiving ensures the information is complete and accurate. This supports better research decisions and faster innovation in healthcare.
6. Facilitating Multi-Center and International Trials
Large clinical trials often involve many research sites in different countries. Coordinating sample handling across these sites can be difficult. Biorepository services solve this by offering centralized storage and tracking. All samples are processed and stored using the same standards. This helps maintain data quality across locations. It also ensures that every site follows the same rules. Standardization reduces errors and simplifies reporting. Central biorepositories allow researchers to access all samples from one place. This improves teamwork and supports faster trial progress on a global scale.
Conclusion
In conclusion, the Clinical Trial Biorepository and Archiving Solutions Market is growing fast due to the rising number of global clinical trials. More researchers now rely on advanced storage systems to keep biological samples safe and usable over time. The shift toward digital archiving, personalized medicine, and strict regulatory standards is shaping the future of this market.
As trials become more complex and decentralized, secure and compliant storage is essential. Biorepositories play a key role in making clinical research more efficient, accurate, and inclusive. With strong support from key players and new technologies, this market is set to expand steadily in the years ahead.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



