Table of Contents
Overview
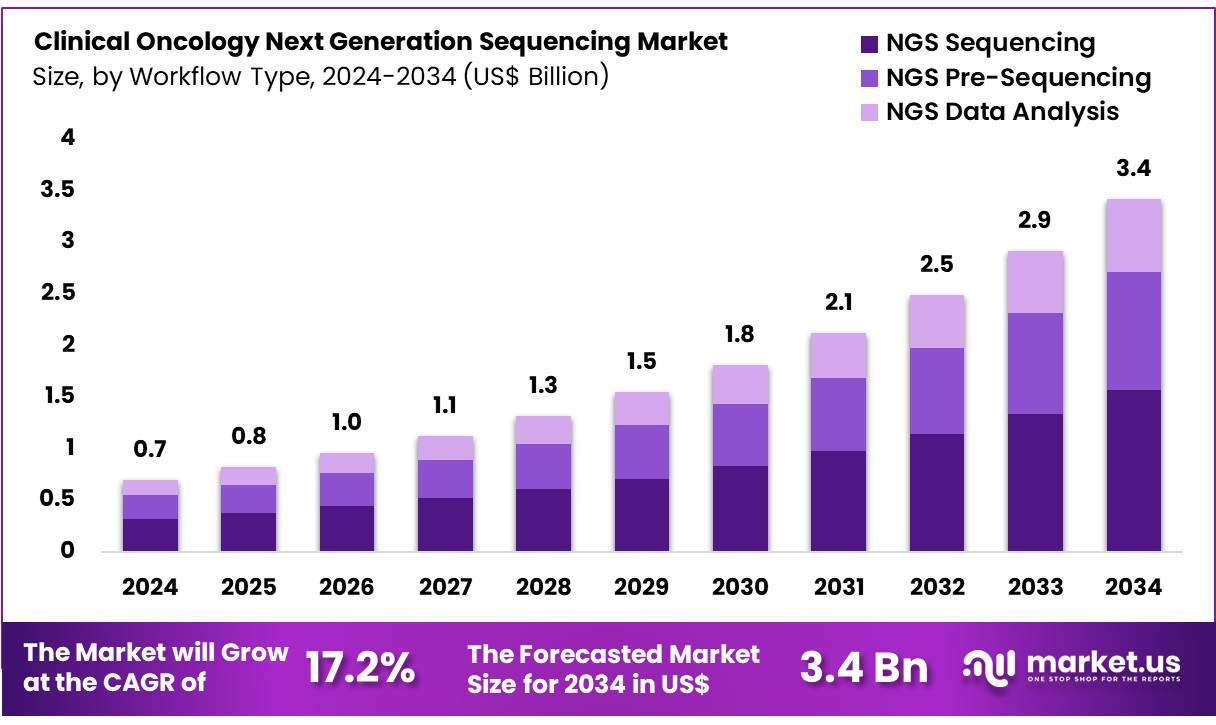
New York, NY – Nov 06, 2025 – Global Clinical Oncology Next Generation Sequencing Market size is expected to be worth around US$ 3.4 Billion by 2034 from US$ 0.7 Billion in 2024, growing at a CAGR of 17.2% during the forecast period 2025 to 2034.
The global Clinical Oncology Next Generation Sequencing market is being shaped by rapid advancements in genomic technologies and the rising demand for precision-based cancer diagnostics. The adoption of sequencing platforms has been strengthened by the ability to deliver high-throughput analysis, enabling the identification of genetic mutations, biomarkers, and therapeutic targets with improved accuracy. The expansion of personalized medicine has encouraged healthcare institutions and research laboratories to integrate sequencing solutions into routine clinical workflows.
Market growth has been supported by the increasing prevalence of cancer and the emphasis on early detection. The adoption of targeted therapies and companion diagnostics has further increased the utilization of sequencing technologies. Continuous reductions in sequencing costs and the availability of streamlined workflows have contributed to broader accessibility across academic centers and hospitals. Strong investments in oncology research have stimulated product innovation, allowing the introduction of more sensitive, faster, and cost-efficient platforms.
North America has maintained a significant market share due to the presence of advanced healthcare infrastructure and supportive regulatory frameworks. The Asia Pacific region is expected to record steady expansion, driven by rising healthcare expenditure and increasing awareness regarding molecular diagnostics. The competitive landscape is characterized by collaborations between technology providers, diagnostic laboratories, and pharmaceutical companies to enhance clinical applications.
Overall, sustained technological innovation and the growing relevance of genomics in clinical decision-making are expected to support the long-term growth of the Clinical Oncology Next Generation Sequencing market.
Key Takeaways
- In 2024, the clinical oncology next generation sequencing market generated revenue of US$ 0.7 Billion, with a CAGR of 17.2%, and is projected to reach US$ 3.4 Billion by 2033.
- By workflow, the market is categorized into NGS sequencing, NGS pre-sequencing, and NGS data analysis, with NGS sequencing leading in 2023 with a share of 45.8%.
- Based on technology, the market includes whole genome sequencing, targeted sequencing and resequencing, and whole exome sequencing, where targeted sequencing and resequencing accounted for 48.6% of the market share.
- By application, the market comprises screening, companion diagnostics, and other uses, with screening holding the largest share at 52.3%.
- In terms of end use, hospitals and clinics and laboratories form the key segments, with laboratories dominating at 57.4% of revenue.
- North America remained the leading regional market, accounting for 41.3% of the global share in 2024.
Segmentation Analysis
- Workflow Type Analysis: In 2023, the sequencing stage held a 45.8% share, supported by its essential function in cancer diagnostics and research. Its ability to generate high-throughput and detailed genomic profiles is vital for identifying tumor-specific mutations. As demand for personalized oncology increases, the accuracy and depth of sequencing data continue to strengthen its adoption, particularly in treatment selection and clinical decision-making across different cancer types.
- Technology Analysis: Targeted sequencing and resequencing accounted for 48.6% of the technology segment in 2023. This approach focuses on selected genomic regions, offering a cost-efficient and precise method for detecting cancer-related mutations. The increasing use of tailored therapies has elevated the importance of targeted sequencing for identifying actionable genetic markers. Its role in supporting mutation-based treatment strategies is expected to drive sustained demand in clinical oncology.
- Application Analysis: The screening category represented 52.3% of market revenue in 2023, reflecting a growing emphasis on early cancer identification. NGS-based screening supports the detection of key biomarkers and genetic alterations at early disease stages, improving therapeutic outcomes. Progress in liquid biopsy and other non-invasive tools is accelerating uptake. Continued movement toward personalized treatment linked to genetic profiles is expected to maintain screening as a leading application area.
- End-Use Analysis: Laboratories held 57.4% of the end-use share in 2023, driven by increased adoption of genomic testing in cancer diagnostics and research. Both hospital and independent laboratories are integrating advanced NGS platforms to enhance diagnostic accuracy and support personalized treatment planning. As precision oncology advances, the need for high-quality genomic data is reinforcing the central role of laboratories in clinical oncology workflows.
Regional Analysis
North America Leading the Clinical Oncology Next Generation Sequencing Market
North America maintained the largest share of the clinical oncology next generation sequencing market in 2023, accounting for 41.3% of global revenue. The dominance of the region can be attributed to the rising incidence of cancer and continuous advancements in precision medicine. According to a 2022 WebMD report, cancer results in more than 600,000 deaths each year in the United States and nearly 80,000 deaths in Canada, demonstrating the growing need for advanced diagnostic and therapeutic solutions.
The increasing adoption of NGS technologies in clinical oncology has enabled the identification of genetic mutations with improved accuracy, supporting the development of personalized treatment strategies. Strong research activities in genomics and active collaborations between biotechnology companies and academic institutions have accelerated the integration of NGS. Supportive regulatory policies and consistent government funding for cancer research have further contributed to market expansion.
The application of artificial intelligence in genomic data interpretation has improved workflow efficiency, encouraging wider use of NGS. Higher awareness among clinicians and patients regarding the value of molecular profiling has increased the demand for NGS-based diagnostic procedures. The presence of well-developed healthcare systems and broad access to advanced technologies across the U.S. and Canada remain key drivers of growth in the region.
Asia Pacific Expected to Register the Highest CAGR
The Asia Pacific region is projected to record the fastest CAGR during the forecast period due to the rising cancer burden and increasing investments in modern diagnostic solutions. Improving healthcare infrastructure in China, India, and Japan is expected to strengthen access to NGS technologies. Government-led programs focused on precision medicine and cancer genomics are likely to support market development.
Collaborations between global sequencing technology providers and regional healthcare organizations are anticipated to increase the availability and cost efficiency of advanced NGS platforms. The expanding middle-class population and rising healthcare spending in emerging economies are expected to fuel the demand for targeted cancer treatments. Growing awareness regarding early diagnosis and personalized therapy is projected to encourage the adoption of NGS-based tools.
Medical tourism supported by affordable cancer care services is also expected to attract patients seeking advanced diagnostic options. Continuous technological improvements in sequencing systems and bioinformatics are likely to enhance the accuracy and performance of NGS, thereby strengthening market growth across the Asia Pacific region.
Frequently Asked Questions on Clinical Oncology Next Generation Sequencing
- How does NGS improve cancer diagnosis?
NGS improves cancer diagnosis by identifying gene mutations, chromosomal changes, and biomarkers associated with tumor development. This detailed genetic information enhances clinical decision-making, supports early detection, and enables personalized treatment strategies based on molecular characteristics of individual cancers. - What types of cancers are commonly analyzed using NGS?
NGS is widely applied across solid tumors and hematological malignancies, including lung, breast, colorectal, ovarian, and leukemia. Its broad utility is attributed to the need for genetic characterization, which supports personalized care and targeted therapeutic interventions. - What are the key advantages of NGS in oncology?
The advantages of NGS include high sensitivity, rapid throughput, cost-effective multi-gene analysis, and the ability to detect rare variants. These capabilities improve diagnostic accuracy and support the adoption of precision medicine in clinical oncology workflows. - Which regions are leading the Clinical Oncology NGS market?
North America and Europe hold dominant market positions due to advanced healthcare infrastructure, strong research investments, and early adoption of genomic technologies. Asia Pacific is experiencing rapid growth driven by expanding diagnostic capabilities and rising healthcare expenditure. - What technologies are commonly used in Clinical Oncology NGS?
Targeted sequencing, whole-exome sequencing, and whole-genome sequencing are widely used technologies. Their selection depends on clinical need, cost considerations, and depth of genomic information required for diagnostic and therapeutic decision-making. - How is NGS used for treatment planning in cancer care?
NGS identifies actionable mutations that inform targeted therapies and immunotherapies. The results support oncologists in selecting treatments that match tumor genetic profiles, improving therapeutic outcomes and reducing exposure to ineffective or unnecessary interventions.
Conclusion
The clinical oncology next generation sequencing market is expected to advance steadily as precision medicine becomes central to cancer care. Growth is being supported by declining sequencing costs, increasing adoption of targeted therapies, and strong investment in genomic research.
Regions such as North America continue to lead due to established healthcare systems, while Asia Pacific is projected to expand rapidly with rising healthcare spending and improved diagnostic infrastructure.
Continuous innovation in sequencing platforms, bioinformatics, and clinical applications is strengthening market adoption. Overall, NGS is anticipated to remain a critical tool in early detection, personalized treatment, and improved cancer management worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



