Table of Contents
Overview
New York, NY – Aug 04, 2025: The Core Clinical Molecular Diagnostics Market is projected to reach US$ 13.5 Billion by 2034. This is up from US$ 5.4 Billion in 2024, growing at a CAGR of 9.6% from 2025 to 2034. This strong growth is driven by the rising need for accurate, fast, and non-invasive diagnostic methods. Healthcare providers are increasingly adopting molecular diagnostic tools. These tools detect diseases at the genetic level, providing high precision. The market is evolving rapidly with technology improvements and broader clinical applications.
Core clinical molecular diagnostics focus on the use of molecular biology in healthcare. These tools help diagnose various conditions like infections, cancer, and genetic disorders. The diagnostics offer higher sensitivity than traditional methods. Their ability to detect diseases in early stages helps improve patient outcomes. Personalized medicine is also becoming more common. With molecular testing, doctors can tailor treatments to the individual. This shift is making diagnostics an essential part of modern healthcare systems worldwide.
The market is seeing increasing demand for point-of-care and home-based diagnostic tools. These solutions help reduce healthcare costs and enhance patient convenience. More patients now prefer home testing for quicker results. Health systems are also adopting these tools to reduce hospital loads. Molecular diagnostics are enabling real-time disease tracking. This is especially useful for chronic conditions and infections. As a result, diagnostic companies are focusing more on developing portable and user-friendly platforms.
Significant public and private investments are boosting market expansion. In May 2023, BARDA committed US$ 53.7 million to Aptitude Medical. This funding supports molecular diagnostics for home and point-of-care use. Such investments show the rising confidence in diagnostic innovations. Funding also helps speed up the development of new tools. These tools are expected to be faster, more accurate, and easier to use. The financial support from government bodies strengthens the industry’s R&D pipeline.
Advancements in molecular technologies will further accelerate market growth. New tools will enhance early detection and monitoring of diseases. These innovations will also support better clinical decision-making. The growing interest in personalized medicine adds to this momentum. As healthcare shifts toward prevention and precision care, diagnostics will play a bigger role. The market will likely see more partnerships and product launches. Overall, core clinical molecular diagnostics are set to transform global healthcare delivery.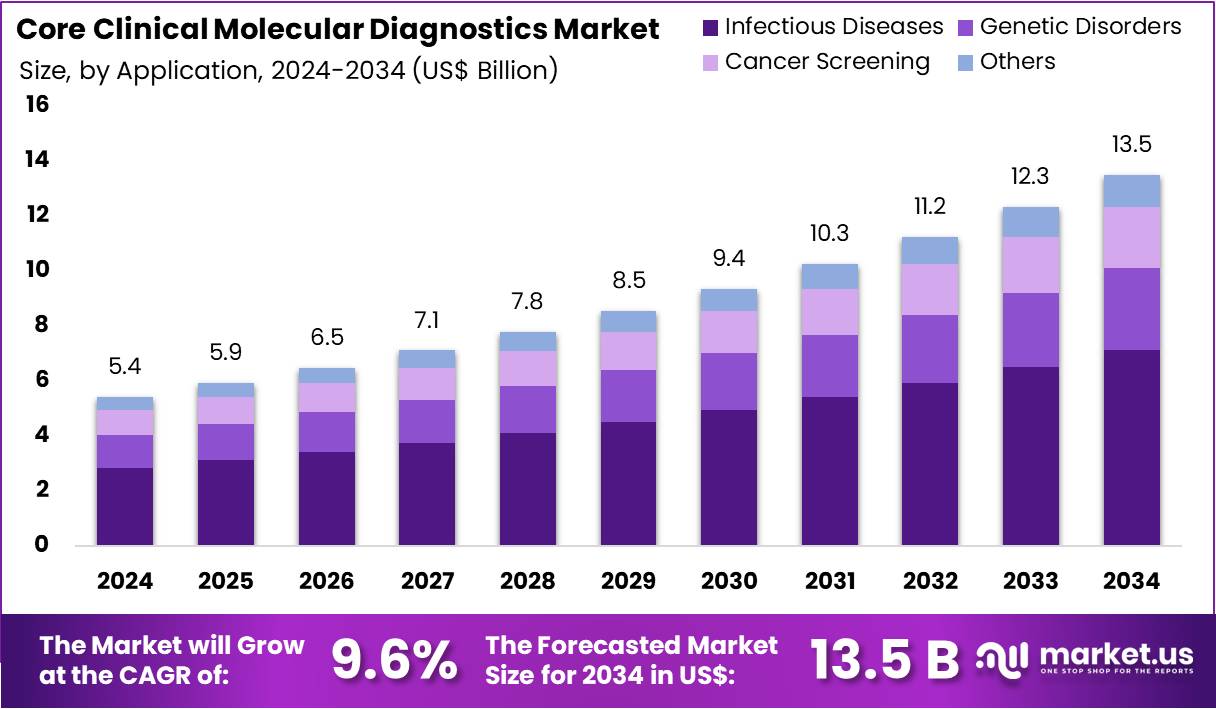
Key Takeaways
- In 2023, the Core Clinical Molecular Diagnostics Market earned US$ 5.4 billion and is projected to hit US$ 13.5 billion by 2033.
- Reagents dominated the product type segment in 2023, capturing 58.5% of the market due to their vital role in molecular testing workflows.
- Infectious diseases led the application segment, accounting for a 52.7% market share, driven by rising demand for fast and accurate pathogen detection.
- PCR emerged as the most used technique in 2023, holding 59.4% of market revenue owing to its speed, accuracy, and diagnostic reliability.
- North America topped regional performance in 2023, contributing 38.8% of total market share due to advanced infrastructure and high R&D investment.
Regional Analysis
North America currently leads the Core Clinical Molecular Diagnostics Market, holding a dominant 38.8% revenue share in 2023. This leadership is largely driven by the growing demand for infectious disease testing. For instance, CDC data shows tuberculosis cases in the U.S. rose by 8%, from 9,622 in 2023 to 10,347 in 2024, highlighting the need for advanced diagnostic tools. Additionally, continuous innovations in molecular testing and favorable FDA approvals are expanding the availability of high-precision diagnostics. The U.S. regulatory framework also supports this growth, with CMS regularly updating coding and reimbursement policies for molecular tests, making diagnostics more accessible and encouraging wider adoption across clinical settings.
The Asia Pacific region is projected to witness the fastest CAGR during the forecast period. This growth is driven by rising healthcare spending, particularly in countries prioritizing universal health coverage, as reported by the OECD. An aging population, as outlined by the United Nations, is also fueling demand for advanced diagnostics tailored to age-related health concerns. Furthermore, the region faces a high burden of infectious diseases—South-East Asia alone accounted for over 45% of global tuberculosis cases in 2023, according to WHO. Government initiatives promoting R&D, healthcare infrastructure, and early disease detection will further accelerate market expansion across Asia Pacific.
Segmentation Analysis
The reagents segment dominated the Core Clinical Molecular Diagnostics Market, securing a 58.5% share. This leadership is attributed to the rising need for accurate and reliable diagnostic tools across healthcare settings. As molecular testing becomes more advanced, high-quality reagents are essential for identifying genetic disorders, infections, and cancers with precision. The growing focus on personalized medicine and chronic disease management is further boosting demand. Additionally, innovations like multiplex assays are enhancing efficiency and expanding the role of reagents in clinical diagnostics.
In terms of application, infectious diseases held the largest share at 52.7% in 2023, driven by the increasing global burden of viral, bacterial, and fungal infections. The emergence of new pathogens has heightened the need for rapid and reliable testing. Molecular diagnostic technologies, particularly PCR and next-generation sequencing, are transforming infectious disease detection by offering faster and more accurate results. Rising healthcare investments and enhanced disease surveillance systems are also contributing to the segment’s growth, as early and precise diagnosis becomes critical to disease control and public health management.
The PCR technique led the market with a 59.4% revenue share, solidifying its position as a cornerstone of molecular diagnostics. PCR is widely valued for its high sensitivity and specificity in detecting genetic material from pathogens, cancer cells, and hereditary conditions. Ongoing advancements in PCR automation and performance are making the technology more efficient and accessible, especially in resource-limited settings. The broader adoption of personalized medicine and the increasing emphasis on early diagnosis particularly for cancer and infectious diseases continue to support the widespread use of PCR in clinical practice.
Key Players Analysis
Leading companies in the core clinical molecular diagnostics market are accelerating growth through strong R&D investments, diversified product pipelines, and expanded global reach. These players are focused on enhancing diagnostic accuracy and scalability by developing advanced platforms, reagents, and bioinformatics tools. Collaborations with academic and healthcare institutions further support technology integration and market adoption. Moreover, expanding into emerging markets with growing healthcare infrastructure and rising disease incidence offers lucrative opportunities for sustained business development.
Thermo Fisher Scientific stands out as a key player, headquartered in Waltham, Massachusetts, with a strong global footprint in molecular diagnostics. The company provides a wide array of products, including PCR and sequencing assays for clinical use. In Q1 2025, Thermo Fisher exceeded expectations by generating $10.36 billion in revenue, fueled by continued demand for its research and clinical tools. With operations in over 50 countries, the company supports sectors ranging from academia to healthcare and continues to grow through strategic acquisitions and innovation-driven product launches.
Emerging Trends
- Shift Toward At-Home Molecular Testing: There is a growing trend toward at-home molecular testing. Many companies are now creating easy-to-use test kits for home use. These kits can detect infections and genetic conditions without visiting a hospital or laboratory. This shift began with the success of at-home COVID-19 tests, which proved fast, reliable, and convenient. Patients prefer the comfort and privacy of testing at home. It also helps reduce pressure on clinics and labs. At-home tests are improving in accuracy and reliability. As the demand for remote healthcare grows, this trend will continue to expand, especially for diseases like flu, STDs, and chronic infections.
- Integration of AI and Machine Learning: Artificial Intelligence (AI) is transforming molecular diagnostics. AI tools can now analyze test data faster and with greater precision. When combined with machine learning, these systems learn from past results to improve accuracy over time. This helps doctors detect diseases earlier and choose better treatments. AI can find patterns in large datasets that human experts may miss. It also reduces the risk of errors and shortens diagnosis time. As healthcare becomes more data-driven, integrating AI into molecular testing is becoming essential. This trend is expected to grow, especially in cancer detection, infectious disease diagnosis, and genetic analysis.
- Multiplex Testing Platforms: Multiplex testing is changing how we diagnose diseases. These advanced tools can detect several diseases at once using a single sample. For example, one test can check for flu, COVID-19, and RSV together. This saves time for both patients and laboratories. It also reduces the cost of testing and speeds up treatment decisions. Multiplex systems are especially useful during flu seasons or pandemics. They help doctors quickly understand the cause of symptoms and act fast. With more labs adopting this technology, multiplex testing is becoming a standard part of clinical diagnostics. It offers greater efficiency and better patient care.
- Miniaturization and Portability: Diagnostic tools are becoming smaller and more portable. This is a big advantage for clinics in rural or low-resource areas. Portable molecular diagnostic devices offer fast and accurate results, just like large lab systems. These compact tools are ideal for mobile health units, remote clinics, and emergency situations. They can be used on-site without needing complex infrastructure. This makes molecular testing more accessible and affordable. Miniaturized systems are also easier to maintain and use. As innovation continues, we’ll see more powerful handheld or tablet-sized diagnostic devices. This trend will help close the healthcare gap in underserved regions.
Use Cases
- Early Cancer Detection: Molecular diagnostics help detect cancer early by identifying changes in a person’s genes. One common method is a liquid biopsy. This test finds tiny DNA fragments from tumors in the blood, sometimes even before symptoms begin. Early detection can improve survival rates by over 70%. This approach allows doctors to start treatment earlier, which often leads to better outcomes. It’s especially helpful for hard-to-detect cancers like lung or pancreatic cancer. These tests are non-invasive and can be repeated easily. As awareness grows, more healthcare providers are using molecular diagnostics for cancer screening in high-risk patients.
- Infectious Disease Management: Molecular diagnostics play a key role in fighting infectious diseases. Hospitals use these tests to quickly find viruses like flu, HIV, and hepatitis. Results often come back within 1 to 2 hours, which means treatment can begin sooner. Fast testing also helps prevent disease spread and reduces the risk of complications. Some hospitals report that rapid molecular tests can cut patient stays by nearly 30%. These tests are more accurate than traditional methods, especially for detecting low levels of infection. During outbreaks, fast diagnostics can save lives and protect entire communities by guiding proper treatment and isolation measures.
- Prenatal and Newborn Screening: Molecular diagnostics are used to check for genetic problems in unborn babies and newborns. These tests can find conditions like cystic fibrosis or spinal muscular atrophy very early. In many cases, treatment can begin within weeks of birth. Early diagnosis means better outcomes and lower risk of complications. These tests are often done with just a small blood or saliva sample. They are safe, fast, and reliable. With more parents and doctors seeking early answers, the demand for molecular tests in prenatal care is growing. This approach supports healthier starts for children with inherited medical risks.
- Pharmacogenomics and Personalized Medicine: Doctors are using molecular diagnostics to create treatment plans based on a patient’s genetic profile. This is called pharmacogenomics. It helps predict how someone will respond to a specific drug. For example, around 10–20% of cancer patients get better results with chemotherapy when it’s tailored to their genes. These tests also reduce harmful side effects and increase the chances of success. Personalized medicine is becoming more common in cancer, mental health, and heart disease treatment. With the help of molecular diagnostics, healthcare is shifting from a one-size-fits-all approach to care that’s truly customized for each person.
FAQs Core Clinical Molecular Diagnostics
1. What is core clinical molecular diagnostics?
Ans:- Core clinical molecular diagnostics refers to medical tests that use DNA, RNA, or proteins to detect diseases at a molecular level. These tests help diagnose infections, genetic disorders, and cancers with high accuracy.
2. How does molecular diagnostics differ from traditional diagnostics?
Ans:- Molecular diagnostics identify diseases based on genetic and molecular markers. Traditional methods rely on symptoms, cultures, or imaging. Molecular diagnostics offer faster, more accurate, and often earlier results.
3. What diseases can be detected using molecular diagnostics?
Ans:- They can detect a wide range of diseases including infectious diseases (like HIV, COVID-19), cancers (like lung or colon cancer), and inherited conditions (like cystic fibrosis).
4. Are molecular diagnostic tests safe?
Ans:- Yes, these tests are generally safe and non-invasive. Most use blood, saliva, or swabs. They pose minimal risk and are approved by regulatory agencies before use.
5. How accurate are molecular diagnostic tests?
Ans:- They are highly accurate. Many tests have sensitivity and specificity above 95%, depending on the disease and technique used.
6. What is the size of the core clinical molecular diagnostics market?
Ans:- The market was valued at US$ 5.4 billion in 2023 and is expected to reach US$ 13.5 billion by 2034, growing at a CAGR of 9.6% from 2025 to 2034.
7. What factors are driving growth in this market?
Ans:- Key drivers include rising demand for early and accurate disease detection, growing infectious disease prevalence, technological advancements, and the rise of personalized medicine.
8. Which region holds the largest market share?
Ans:- North America leads the market with a 38.8% share in 2023, due to strong healthcare infrastructure, innovation, and regulatory support.
9. Which segment dominates the market by product type?
Ans:- Reagents held the largest share at 58.5% in 2023, owing to their essential role in testing processes across various applications.
10. What is the fastest-growing application segment?
Ans:- Infectious diseases represent the leading application segment, holding a 52.7% share due to the global focus on rapid infection detection.
Conclusion
The Core Clinical Molecular Diagnostics Market is experiencing rapid growth, projected to more than double from US$ 5.4 billion in 2024 to US$ 13.5 billion by 2034 at a strong CAGR of 9.6%. This expansion is driven by increasing demand for accurate, early, and personalized disease detection across a wide range of conditions including cancer, infections, and genetic disorders.
Innovations like AI integration, multiplex testing, and portable diagnostics are reshaping how and where testing is delivered making it faster, more accessible, and patient friendly. With strong R&D investments, rising healthcare needs, and growing adoption of precision medicine, molecular diagnostics are becoming a central pillar of global healthcare advancement.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



