Table of Contents
Overview
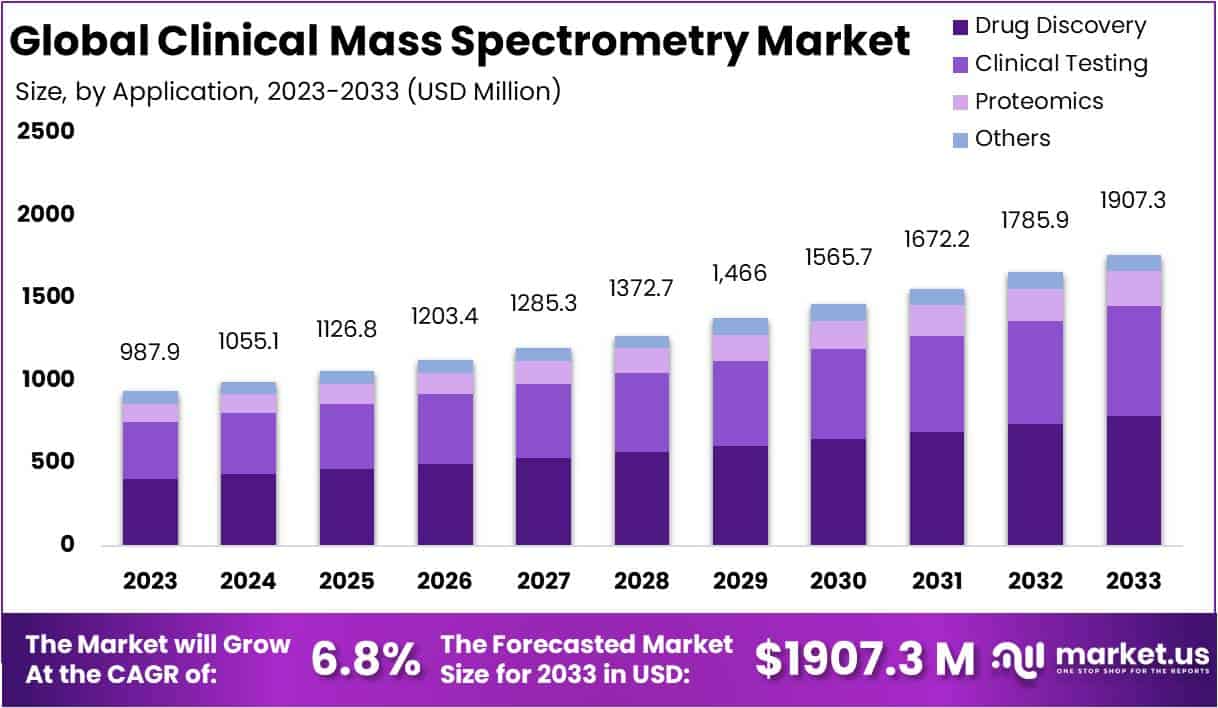
The Global Clinical Mass Spectrometry Market is projected to reach US$ 1,907.3 million by 2033, up from US$ 987.9 million in 2023, growing at a CAGR of 6.8% between 2024 and 2033. This growth is being driven by the rising demand for fast and accurate diagnostics. Evidence from global health systems shows that laboratory results guide most clinical decisions, while representing only a small share of hospital budgets. Reducing diagnostic errors is therefore a major incentive for adopting precise technologies such as LC-MS/MS and MALDI-TOF.
Regulatory clearances are further supporting this expansion. The U.S. FDA has cleared several MALDI-TOF systems for clinical use, allowing validated and reliable workflows for identifying bacteria and fungi in patient samples. These approvals reduce the risks associated with adoption and encourage hospitals and laboratories to invest in mass spectrometry platforms. This regulatory confidence is improving trust in the technology and accelerating its acceptance in mainstream clinical diagnostics worldwide.
Another major driver of demand is the long-standing use of newborn screening programs. Guidance from the CDC highlights how tandem mass spectrometry can detect a wide range of metabolic disorders from a single dried blood spot. This early and reliable detection capability has ensured stable demand for instruments, reagents, and related training. The recurring use of LC-MS/MS in public health programs demonstrates the technology’s reliability and secures consistent funding for its continued adoption.
Structured training and standard validation processes are also reducing barriers for laboratories. The CDC has developed detailed training protocols that assist labs in building in-house capabilities. These include method development, assay validation, and clinical reporting. As more laboratories adopt standardized workflows, the challenge of limited technical expertise is reduced. This is helping laboratories across both developed and developing regions to expand their diagnostic capabilities using mass spectrometry.
Expanding Applications and Future Outlook
Clinical mass spectrometry is gaining importance in antimicrobial resistance (AMR) management. Reports from the WHO and ECDC highlight the role of MALDI-TOF in rapidly identifying organisms and supporting hospital infection control programs. Countries are integrating these systems into AMR action plans, unlocking funding and policy-level backing for widespread adoption. By accelerating pathogen identification, mass spectrometry contributes directly to public health strategies aimed at reducing the risks associated with resistant infections.
Operational advantages are further strengthening market growth. MALDI-TOF is recognized for its fast turnaround times and lower per-test costs, compared to some conventional diagnostic methods. WHO country reports emphasize its high specificity, faster workflows, and reduced consumable costs, making it a cost-effective option for budget-constrained laboratories. While ongoing validation remains necessary for certain organisms, the financial and efficiency benefits of these platforms are driving broader adoption across healthcare systems.
The role of mass spectrometry is also expanding in biotherapeutics and vaccine quality control. Technical reports from the WHO reference LC-MS and MALDI-TOF methods in the detailed characterization of monoclonal antibodies and vaccines. As regulatory standards for biologics become more demanding, laboratories connected to public health and clinical trials are increasing their investment in MS systems. This trend is expected to broaden the market beyond patient diagnostics and into pharmaceutical quality analytics.
The precision medicine agenda is creating a pipeline of new opportunities. Targeted proteomics and MS-based biomarker discovery are being actively validated in clinical research, with platforms being adapted to generate reproducible, “clinical-grade” data. This activity is ensuring steady demand for robust MS platforms. Combined with OECD evidence on the economic burden of misdiagnosis, hospitals and governments are prioritizing procurement of mass spectrometry systems. By improving diagnostic accuracy and supporting long-term healthcare efficiency, these systems are positioned as a core technology in future diagnostic pathways.
Key Takeaways
- The Clinical Mass Spectrometry market is projected to achieve USD 1,907.3 million by 2033, registering a strong CAGR of 6.8% during 2024–2033.
- Gas Chromatography Mass Spectrometry (GC-MS) accounted for over 30.9% market share in 2023, highlighting its reliability and established position among available technologies.
- Drug Discovery led applications with a 41.2% market share in 2023, underscoring its critical role in advancing pharmaceutical research and innovation pipelines.
- Hospitals held the largest share of 58.2% in 2023, confirming their central role as primary end users of clinical mass spectrometry solutions.
- North America dominated with a 37.6% market share and USD 371.4 million value in 2023, reflecting strong adoption and healthcare infrastructure advancement.
- Technological trends emphasize miniaturization and integration of instruments, enabling point-of-care testing and driving widespread adoption across diverse healthcare and diagnostic settings.
- Rising mergers, acquisitions, and licensing agreements are strengthening industry capabilities, accelerating innovation, and consolidating leadership among key players in clinical mass spectrometry.
Regional Analysis
In 2023, North America held the leading position in the Clinical Mass Spectrometry Market with a notable 37.6% share, valued at USD 371.4 million. This dominance was driven by the region’s advanced healthcare infrastructure and the presence of leading pharmaceutical companies. Strong diagnostic capabilities, cutting-edge research institutions, and established clinical practices supported the adoption of mass spectrometry technologies. The region’s consistent focus on improving patient care and precision diagnostics further strengthened its ability to remain the frontrunner in this competitive global market.
A major factor supporting growth in North America is its substantial investment in research and development activities. Continuous innovation, rapid technological advancements, and adoption of next-generation clinical solutions have accelerated market expansion. The presence of collaborative networks between academia, healthcare providers, and industry players has created a strong innovation ecosystem. Government initiatives and funding programs directed toward healthcare modernization have also contributed significantly. These combined elements have positioned North America as a hub for technological breakthroughs in clinical mass spectrometry applications.
Additionally, the availability of highly skilled professionals and supportive regulatory frameworks has simplified the integration of advanced clinical mass spectrometry tools. Clear guidelines for diagnostic approval, combined with strong compliance standards, have enhanced adoption across hospitals, laboratories, and research centers. The active role of regulatory agencies in encouraging advanced diagnostic solutions has created confidence among stakeholders. Furthermore, favorable reimbursement policies and high awareness of innovative diagnostic approaches continue to drive market demand. These strengths firmly establish North America’s leadership in clinical mass spectrometry technologies.
Segmentation Analysis
In 2023, Gas Chromatography Mass Spectrometry (GC-MS) dominated the clinical mass spectrometry market, securing a leading share of over 30.9%. This position was driven by its proven effectiveness in analyzing complex mixtures, making it the preferred choice in diagnostics and research. Liquid Chromatography Mass Spectrometry (LC-MS) followed closely, benefiting from its versatility and high sensitivity across diverse compounds. Additionally, MALDI-TOF Mass Spectrometry gained steady traction for its rapid identification of biomolecules, proving essential in microbial analysis and protein characterization within healthcare applications.
Applications also shaped the market significantly in 2023. The Drug Discovery segment held the largest share at 41.2%, highlighting mass spectrometry’s pivotal role in identifying and analyzing potential drug candidates. Clinical Testing accounted for a notable share, supported by the rising demand for precise diagnostic tools. Proteomics strengthened its position by enabling researchers to decode protein structures and functions. Meanwhile, other applications, including food safety and environmental testing, underscored the technology’s adaptability and wide-ranging importance across industries.
End-user dynamics highlighted hospitals as the most dominant segment, capturing more than 58.2% share in 2023. This leadership underscored their reliance on mass spectrometry technologies for advanced clinical applications. Diagnostic Centers contributed a strong share as well, supported by growing adoption for efficient and accurate testing. Research Laboratories also played a critical role, driving innovation and scientific exploration. The “Other” category, comprising specialized healthcare facilities, reflected the technology’s broader integration. Collectively, these end users accelerated advancements in diagnostics, research, and personalized medicine.
Key Market Segments
Product Type
- Gas chromatography Mass Spectrometry
- Liquid chromatography Mass Spectrometry
- MALDI-TOF Mass Spectrometer
- Capillary Electrophoresis Mass Spectrometry
- Ion Mobility Spectrometry
Application
- Drug Discovery
- Clinical Testing
- Proteomics
- Others
End User
- Hospitals
- Diagnostic Centers
- Research Laboratories
- Others
Key Players Analysis
In the clinical mass spectrometry market, Thermo Fisher Scientific Inc. stands out with its advanced technological expertise and strong focus on innovation. The company’s dedication to delivering cutting-edge solutions and quality standards has positioned it as a major industry influencer. Danaher follows closely, with a diversified portfolio and strong emphasis on research and development. This strategic approach allows it to adapt effectively to changing market demands, thereby gaining a competitive advantage. Both companies significantly shape innovation and competition within the clinical mass spectrometry landscape.
Agilent Technologies plays a crucial role through its precision instruments and analytical solutions. Its customer-centric approach helps address the evolving needs of the clinical sector, improving efficiency in diagnostic applications. PerkinElmer Inc. further strengthens the market by emphasizing healthcare innovation. The company focuses heavily on research and technological progress, equipping clinicians with powerful diagnostic and research tools. Together, these players provide reliable solutions that enhance clinical research outcomes and drive sustainable market growth in the healthcare sector.
Bruker has earned recognition for its state-of-the-art mass spectrometry systems, consistently advancing analytical performance. Shimadzu Corporation further contributes to market competitiveness by offering versatile systems tailored for clinical use. Other notable players include Kore Technologies, BME Bergmann, Mass Spectrometry Instruments, and Photonics GmbH, all of whom add diversity to the industry landscape. Collectively, these companies drive technological advancements, foster competition, and strengthen the global clinical mass spectrometry market, creating a robust ecosystem that benefits clinicians, researchers, and patient care outcomes.
Market Key Players
- Thermo Fisher Scientific Inc.
- Danaher
- Agilent Technologies
- PerkinElmer Inc
- Bruker
- Shimadzu Corporation
- Kore Technologies
- BME Bergmann
- Mass Spectrometry Instruments
- Photonics GmbH
Conclusion
The clinical mass spectrometry market is moving toward strong and steady growth, supported by advances in healthcare systems, regulatory approvals, and broader applications. Its role in accurate diagnostics, newborn screening, and antimicrobial resistance management has made it an essential tool in modern laboratories. The technology also brings financial and operational advantages, making it a practical choice for hospitals and research centers. With expanding use in precision medicine, pharmaceutical quality control, and public health programs, clinical mass spectrometry is positioned as a core diagnostic technology for the future. Growing investments, structured training, and strong industry participation will continue to strengthen its adoption and long-term market outlook.
View More
Mass Spectrometry Market || Spectrometry Market || Mass Spectrometry Imaging Market || Proteomics Market || Metabolomics Market || Metabolic Testing Market || ADME-Toxicology Testing Market || AI In Predictive Toxicology Market || Genetic Toxicology Testing Market || Nutrigenomics Market || AI In Genomics Market || Genomics Market || Consumer Genomics Market || Pharmacogenomics Market || Epigenomics Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



