Introduction
The Global Clinical Mass Spectrometry Market is projected to grow from USD 987.9 million in 2023 to USD 1907.3 million by 2033, at a CAGR of 6.8%. This growth is spurred by technological advancements that enhance applications in biomedicine among other fields. A notable innovation, fragment correlation mass spectrometry developed by Stanford, revolutionizes protein analysis by enabling rapid identification without pre-separation, essential for proteomics and personalized medicine.
Incorporating nanotechnology, researchers at Brown University have devised a method that transfers ions directly into a mass spectrometer’s vacuum. This bypasses traditional drying droplets, enhancing the sensitivity and efficiency of analyses, especially in proteomics where small sample sizes are common. This technological leap forward reduces the need for extensive vacuum systems.
Collaboration across disciplines further accelerates growth in this sector. For example, the Mayo Clinic integrates clinical mass spectrometry with translational science programs, connecting researchers with clinical trials. This integration speeds the transition from laboratory discoveries to medical treatments and improves disease understanding through precise molecular analysis.
Additionally, platforms like the Global Natural Products Social Molecular Networking (GNPS) facilitate data sharing and analysis globally, boosting molecule discovery and collaborative research. These platforms exemplify the sector’s move towards a more interconnected scientific community.
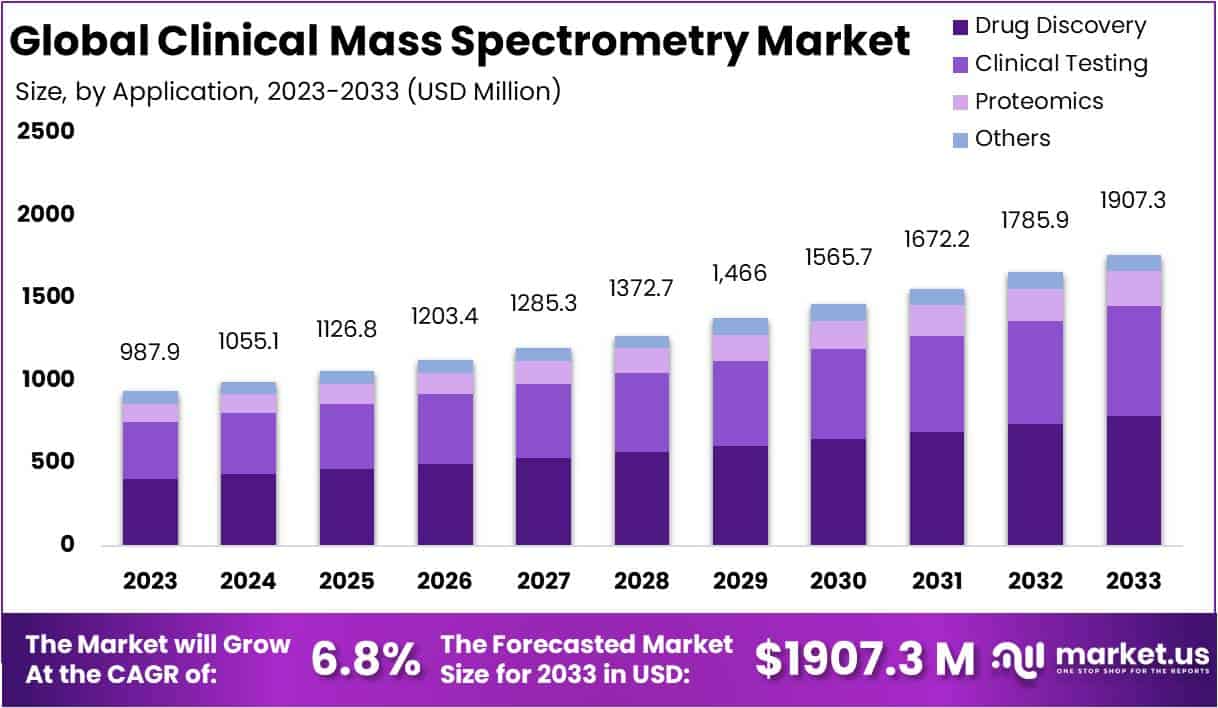
Key Takeaways
- The Clinical Mass Spectrometry market is projected to hit USD 1,907.3 million by 2033, expanding at a 6.8% CAGR from 2024-2033.
- Gas Chromatography Mass Spectrometry holds a substantial 30.9% market share in 2023, highlighting its significant utility.
- Drug discovery leads application sectors with a robust 41.2% market share in 2023, emphasizing its critical importance.
- Hospitals lead end user segments, holding a dominant 58.2% market share in 2023, reflecting their essential role.
- In 2023, North America leads the global market with a 37.6% share, valued at USD 371.4 million.
- Technological advancements focus on shrinking and integrating mass spectrometry instruments for enhanced point-of-care testing.
- The market is witnessing growth through increased mergers, acquisitions, and licensing, driving innovation and enhancing capabilities.
Regional Analysis
In 2023, North America secured a leading position in the Clinical Mass Spectrometry Market, holding a 37.6% share. This translates to a market value of USD 371.4 million. The region’s dominance is underpinned by its sophisticated healthcare infrastructure. This includes top-tier healthcare facilities, advanced research centers, and a robust pharmaceutical sector. These elements create a favorable environment for integrating clinical mass spectrometry technologies, which are pivotal for advancing medical research and diagnostics.
The region’s commitment to medical research and diagnostic innovation plays a crucial role in its market leadership. North America is known for its proactive approach to adopting cutting-edge technologies in healthcare. This enhances the quality of clinical diagnostics and research outcomes. The continuous push for innovation is a key driver of North America’s commanding presence in the global clinical mass spectrometry landscape.
Significant investments in research and development characterize the North American market. These investments drive ongoing technological advancements in clinical mass spectrometry. The region’s market benefits from collaborations between academia and industry, which foster innovation. Additionally, government initiatives aimed at supporting healthcare innovation are pivotal in maintaining the region’s market leadership.
The presence of skilled professionals and a supportive regulatory framework in North America aids the implementation of clinical mass spectrometry solutions. This ensures a smooth integration of advanced technologies in healthcare systems. Moreover, the strong regulatory support helps maintain high standards in healthcare services, contributing to the region’s strong market position.
Emerging Trends
- Increasing Use of Therapeutic Drug Monitoring: Therapeutic Drug Monitoring (TDM) is becoming a critical application of clinical mass spectrometry. Hospitals are now using this technology to quickly measure drug levels in patient systems. This capability facilitates rapid adjustments in dosages, enhancing the effectiveness of treatments and improving patient outcomes. As a result, clinical decisions can be made more swiftly, ensuring that patients receive the most appropriate therapeutic interventions at the right times.
- Advancements in Automation and Integration: In the realm of clinical mass spectrometry, there is a noticeable shift towards automation. This development is highlighted by the introduction of semi-automated and fully automated LC-MS/MS systems. These innovations aim to boost efficiency, reduce the reliance on manual labor, and streamline operations within clinical laboratories. By enhancing the accessibility and functionality of mass spectrometry, these systems are setting new standards for routine diagnostic procedures.
- Transition to FDA-approved Systems: Clinical settings are progressively moving from open-access LC-MS/LDT systems to FDA-approved automated platforms. This transition addresses several challenges, including throughput limitations and the high costs associated with manual methods. FDA-approved systems provide the needed selectivity and specificity but with greater efficiency and lower labor requirements, making them a valuable asset in clinical diagnostics.
- Focus on Direct Sampling Techniques: There is a growing emphasis on direct sampling techniques in clinical mass spectrometry. These methods minimize the need for extensive sample preparation, leading to faster and more efficient analysis. This is particularly advantageous in clinical diagnostics, where quick decision-making is essential. Direct sampling enhances the ability to perform rapid tests, providing critical data that can significantly influence patient care strategies.
Use Cases
- Drug Testing: Clinical mass spectrometry, particularly LC-MS/MS, is a cornerstone in drug testing. It’s renowned for its precision, playing a pivotal role in measuring drug concentrations within patients’ bloodstreams. This level of accuracy is essential for guiding therapy management, ensuring patients receive the correct dosage for optimal therapeutic effects.
- Monitoring Vitamin D Levels: Another vital application of LC-MS/MS in clinical settings is the measurement of 25-hydroxyvitamin D. Accurate monitoring of these levels is critical for diagnosing and managing vitamin D deficiency. This deficiency can lead to bone disorders among other health issues, making precise measurements crucial for patient care.
- Neonatal Screening: In neonatal care, LC-MS/MS is utilized for screening newborns for metabolic disorders. Early detection through this technology is essential as it enables immediate treatment, which is crucial in preventing long-term health complications in infants. This early intervention can significantly improve the quality of life for affected newborns.
- Identifying Monoclonal Immunoglobulins: LC-MS/MS is also employed in identifying and quantifying monoclonal immunoglobulins. These proteins are critical markers for diagnosing and monitoring conditions such as multiple myeloma. Accurate quantification helps in assessing the progression of such diseases and tailoring individualized treatment plans.
Conclusion
In conclusion, the Clinical Mass Spectrometry market is poised for significant growth, driven by innovative technological advancements and interdisciplinary collaborations. These developments are enhancing the precision and efficiency of medical diagnostics and research, with notable contributions from automation and integration of mass spectrometry systems. North America leads this dynamic field, benefiting from robust healthcare infrastructure and significant investments in research and development. Emerging trends, such as therapeutic drug monitoring and the adoption of FDA-approved systems, further underscore the critical role of clinical mass spectrometry in improving patient outcomes and facilitating quick, accurate medical decisions. As the market continues to evolve, these innovations are expected to expand the applications and effectiveness of clinical mass spectrometry in healthcare.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



