Table of Contents
Introduction
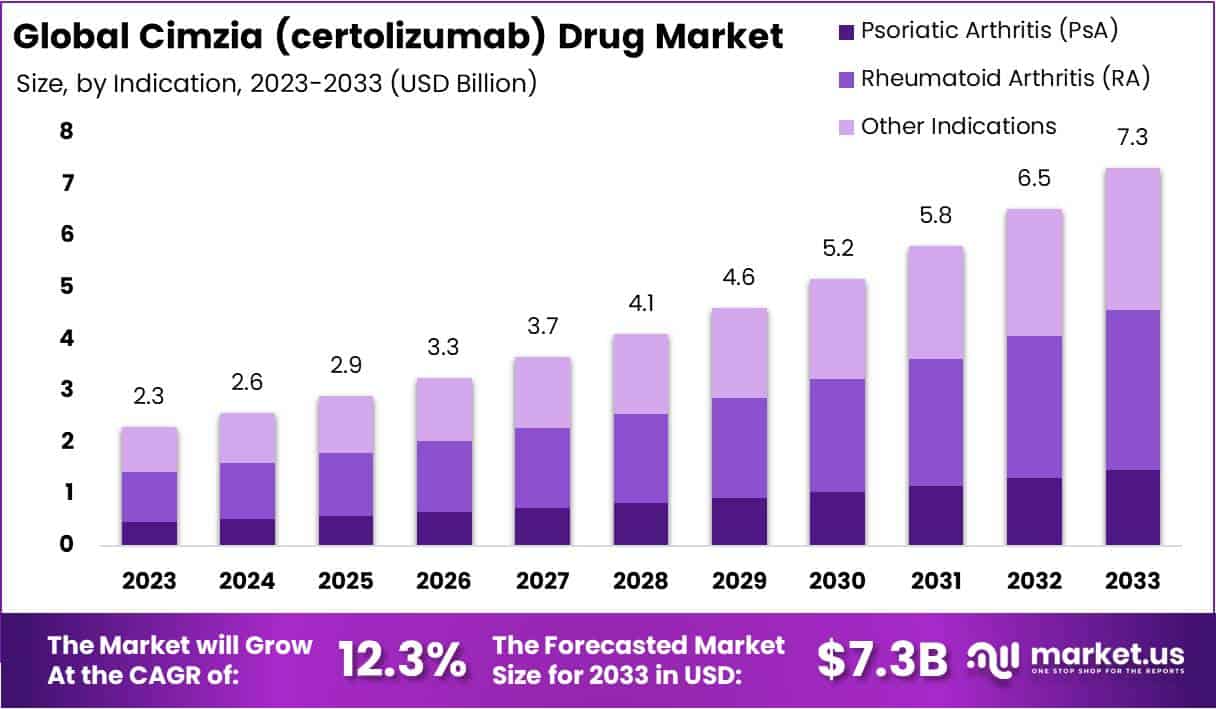
The Global Cimzia (certolizumab) Drug Market is projected to expand significantly, reaching an estimated USD 7.3 billion by 2033 from USD 2.3 billion in 2023, at a CAGR of 12.3%. This growth is driven by the drug’s approval for multiple conditions such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn’s disease, enabling it to access a wider patient demographic.
Clinical trials have underscored Cimzia’s efficacy, particularly the RAPID-axSpA study for axial spondyloarthritis, demonstrating substantial benefits over placebos in symptom improvement and physical function. These successful outcomes bolster the drug’s credibility and foster its integration into clinical practice.
While Cimzia’s expansion into pediatric uses is under discussion, specifics remain limited. Nevertheless, potential label extensions could tap into new market segments. However, the drug’s management requires vigilance due to possible serious side effects, including infections and exacerbation of other conditions, necessitating regular patient monitoring.
Continued research and updates on Cimzia’s interactions and long-term effects remain crucial for enhancing safety and maintaining its competitive market stance. This ongoing commitment to research supports the drug’s sustained market presence and future growth potential.
Key Takeaways
- The Cimzia market is projected to hit USD 7.3 billion by 2033, with an annual growth rate of 12.3% from 2024.
- Cimzia is essential for treating rheumatoid arthritis, Crohn’s disease, and psoriatic arthritis by inhibiting TNF-alpha.
- In 2020, global sales of Cimzia exceeded $6 billion, boosting the TNF-alpha inhibitor market.
- The rheumatoid arthritis segment dominated in 2023, holding a 42.3% share of the market.
- Hospital pharmacies lead distribution with 46.5% market share; retail and online pharmacies follow.
- Market growth is fueled by increasing autoimmune diseases and environmental factors.
- Challenges like high costs and access issues hinder the market’s expansion.
- Emerging markets present significant growth opportunities for Cimzia.
- New developments in biologic drug delivery systems are improving patient compliance and treatment results.
- North America is the leading region with a 60.5% market share, followed by Europe and Asia-Pacific.
Regional Analysis
North America Leads the Cimzia Drug Market in 2023
In 2023, North America dominated the Cimzia (certolizumab) drug market, capturing over 60.5% of the market share with a valuation of USD 1.39 billion. This leadership stems from advanced healthcare infrastructure, extensive patient education programs, and favorable reimbursement policies. The region benefits from significant R&D initiatives by pharmaceutical giants, ensuring continuous innovation. Such factors contribute to the widespread adoption of Cimzia for autoimmune disorders. North America’s dominance underscores the importance of strategic healthcare investments and patient-focused initiatives in driving market growth.
Europe Secures Second Position in Market Share
Europe emerged as the second-largest market for Cimzia in 2023, driven by the rising prevalence of autoimmune diseases like rheumatoid arthritis and Crohn’s disease. The region’s robust healthcare systems and regulatory frameworks facilitate access to advanced treatments. Patient-centric programs and early diagnosis efforts further enhance market growth. Pharmaceutical companies in Europe also benefit from supportive government policies. These factors collectively position Europe as a strong contender in the global Cimzia market, maintaining steady growth momentum.
Asia-Pacific Exhibits the Fastest Growth Potential
The Asia-Pacific region is projected to grow rapidly in the Cimzia drug market. Improvements in healthcare facilities, rising healthcare expenditures, and increasing awareness of autoimmune diseases fuel this growth. Governments across the region are prioritizing healthcare advancements, creating opportunities for drug manufacturers. The introduction of generic versions of Cimzia is expected to make treatments more affordable and accessible. These developments point to a promising future for the Cimzia market in Asia-Pacific, making it a key growth area globally.
Modest Growth in Latin America and MEA Regions
Latin America and the Middle East & Africa are expected to experience modest growth in the Cimzia drug market. Efforts to improve healthcare services, expand government healthcare initiatives, and establish partnerships with international firms are key drivers. Local and global pharmaceutical collaborations are enhancing access to advanced treatments. While these regions currently represent smaller market shares, ongoing developments in healthcare reach and infrastructure signal steady progress. This growth complements the global outlook for Cimzia, which remains optimistic due to continued R&D and strategic partnerships.
Challenges
- High Costs and Accessibility Issues: Cimzia’s significant cost presents a major challenge. Its affordability is particularly problematic in less economically developed regions or in healthcare systems that are underfunded. The expense limits the drug’s accessibility, making it difficult for patients who need this medication but do not have adequate financial resources or health coverage. This issue underscores the need for strategies that can help reduce costs and improve patient access to this important treatment.
- Patent Expiration and Market Competition: The patent for Cimzia is set to expire in 2024 in the United States. This expiration opens the market to biosimilars, which are similar to original biologic drugs but typically offered at lower prices. The entry of these biosimilars could lead to increased competition, potentially driving down the price of Cimzia. This change in the market dynamics poses a challenge for the original manufacturers, as they will need to navigate the new competitive landscape and possibly adjust their pricing strategies.
Opportunities
- Expanding into Emerging Markets: Emerging markets offer immense growth potential for Cimzia. Regions like Asia-Pacific, Latin America, and the Middle East & Africa are improving their healthcare systems. These areas are witnessing increased healthcare spending and better infrastructure. Expanding into these markets can help reach underserved populations. Additionally, rising awareness about autoimmune diseases in these regions further supports growth opportunities. By targeting these markets, companies can strengthen their global presence and tap into high-growth areas.
- Introduction of Generics and Biosimilars: The expiration of patents creates room for introducing generics and biosimilars. These alternatives can enhance market accessibility by reducing costs. Generic versions of Cimzia could increase competition but also broaden patient access. Lower costs can make the drug more affordable for patients worldwide. This shift allows healthcare providers to offer effective treatments to more people, particularly in cost-sensitive markets. It also aligns with the growing demand for cost-efficient healthcare solutions.
- Rising Demand from Autoimmune Diseases: The global prevalence of autoimmune diseases is on the rise. Conditions like rheumatoid arthritis, Crohn’s disease, and psoriatic arthritis are driving the need for effective treatments. Cimzia is well-positioned to meet this growing demand. With increasing diagnosis rates and patient awareness, the drug’s market potential is expanding. Additionally, advancements in diagnostics and disease management support the adoption of Cimzia. This trend presents a significant opportunity for market growth and patient impact.
Market Trends
- Technological Advancements: Biotechnology innovations are transforming the development of biologic drugs like Cimzia. These advancements are enabling improved formulations and delivery methods, making treatments more efficient and effective. Enhanced drug delivery systems could help reduce dosing frequency and improve patient comfort. As technology progresses, the focus on improving patient compliance is becoming a priority. These innovations may also lead to better outcomes by ensuring the drug reaches the targeted areas more effectively. Such developments are likely to enhance the overall therapeutic impact of Cimzia.
- Shift Towards Personalized Medicine: The healthcare sector is moving toward personalized medicine, which benefits drugs like Cimzia. This trend focuses on tailoring treatments to individual patient needs and conditions, optimizing therapeutic results. By using biomarkers and genetic profiling, personalized medicine ensures more precise and efficient use of TNF-alpha inhibitors like Cimzia. This approach minimizes side effects while improving efficacy. As this trend grows, it could expand the market for targeted therapies, making treatments more accessible and impactful for patients with specific conditions.
Conclusion
The Cimzia (certolizumab) drug market shows strong potential for growth, driven by its effectiveness in treating autoimmune diseases and advancements in biologic drug development. North America leads the market, supported by robust healthcare systems and R&D initiatives, while emerging markets in Asia-Pacific and other regions offer significant expansion opportunities. Challenges like high costs and increasing competition from biosimilars remain, but these are balanced by rising demand for targeted therapies and innovations in drug delivery systems. The focus on personalized medicine further strengthens Cimzia’s relevance by enhancing treatment precision and patient outcomes. Overall, Cimzia remains well-positioned to address the growing global need for effective autoimmune treatments while adapting to evolving market dynamics.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



