Overview
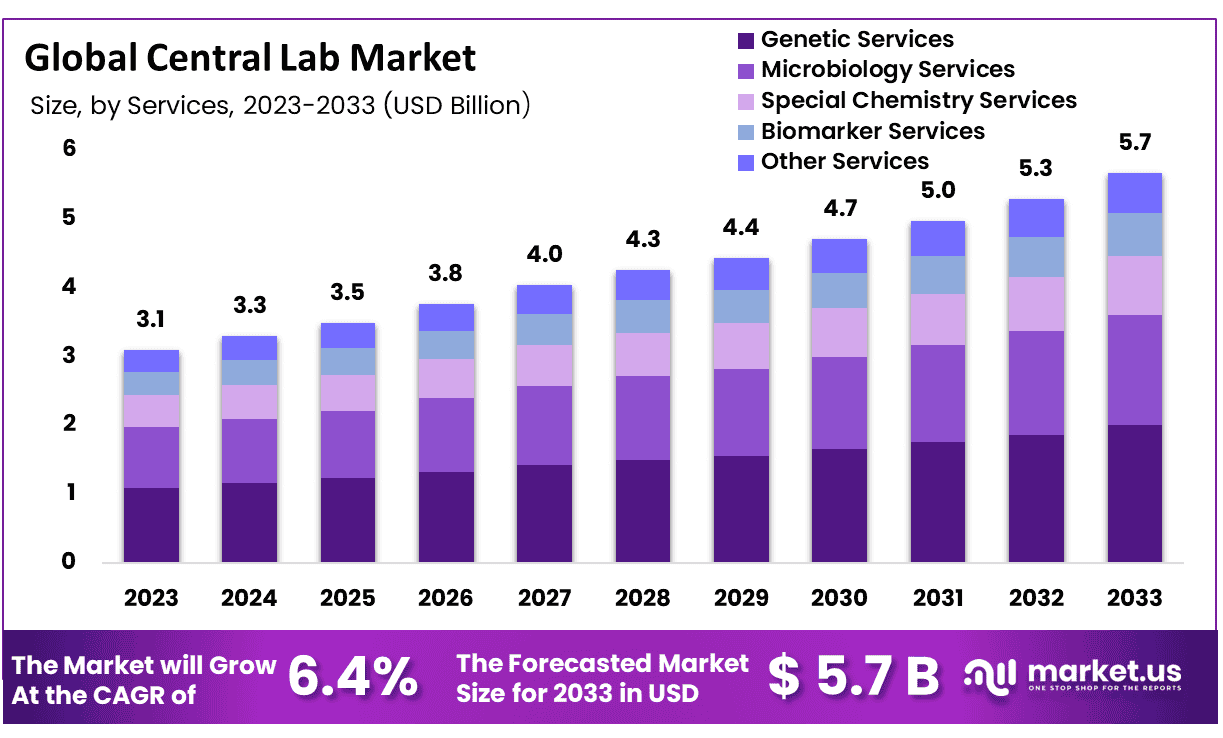
New York, NY – Sep 26, 2025 –The Global Central Lab Market size is expected to be worth around USD 5.7 Billion by 2033 from USD 3.3 Billion in 2024, growing at a CAGR of 6.4% during the forecast period from 2025 to 2033
The establishment of the Central Laboratory marks a significant advancement in strengthening diagnostic, research, and quality assurance capabilities in the region. Designed as a state-of-the-art facility, the Central Lab is equipped with advanced technologies and standardized processes to deliver accurate, reliable, and timely results across multiple domains.
The laboratory has been founded with the objective of serving as a central hub for clinical diagnostics, specialized testing, and scientific innovation. Its infrastructure has been developed in accordance with global best practices, ensuring adherence to rigorous quality standards and regulatory guidelines. The facility is staffed by highly trained professionals, supported by automated systems and digital platforms that enhance operational efficiency.
By consolidating resources under one roof, the Central Lab will enable faster turnaround times, streamlined workflows, and enhanced accessibility for healthcare institutions, research organizations, and industry partners. Specialized units within the lab will focus on molecular diagnostics, microbiology, biochemistry, hematology, and advanced imaging, thereby expanding the scope of services available to stakeholders.
The creation of the Central Lab demonstrates a strong commitment to improving public health outcomes, supporting clinical decision-making, and advancing research. With its strategic vision, the laboratory is expected to play a critical role in fostering collaboration, promoting innovation, and ensuring excellence in diagnostics and scientific development.
Key Takeaways
- Market Size and Growth: The Central Lab market is projected to reach USD 5.7 billion by 2033, up from USD 3.1 billion in 2023, reflecting a compound annual growth rate (CAGR) of 6.4% during the forecast period.
- Service Analysis: Within the service segment, biomarker services emerged as the leading category, contributing 37.6% of total revenue in 2023.
- End-User Analysis: By end user, the pharmaceutical companies’ segment held the dominant position in 2023, accounting for 45% of the global market share.
- Regional Analysis: Regionally, North America represented the largest share, with 38.7% of the market and a valuation of USD 1.2 billion in 2023, underscoring its strong role in the global central lab industry.
- Technological Integration: The adoption of automation, digital pathology, and artificial intelligence (AI) is significantly improving diagnostic accuracy, efficiency, and scalability in central lab operations.
- Regulatory Compliance: Compliance with stringent standards such as CLIA (Clinical Laboratory Improvement Amendments) and CAP (College of American Pathologists) remains a critical factor influencing operational frameworks and market dynamics.
- Challenges and Opportunities: The market faces challenges related to high technology implementation costs and the complexities of managing multi-site clinical trials. However, expanding prospects in personalized medicine and emerging markets present notable opportunities for future growth.
Regional Analysis
In 2023, the North American region accounted for 38.7% of the global central labs market, representing a market value of USD 1.2 billion. Growth in this region has been supported by rapid technological advancements, a well-established healthcare infrastructure across the United States and Canada, and the presence of numerous leading pharmaceutical companies. In addition, the streamlined approval processes for new drugs have further accelerated market expansion in North America.
Looking ahead, the Asia Pacific region is anticipated to register the fastest growth during the forecast period. This expansion can be attributed to the increasing number of clinical studies in China, coupled with a steady rise in the establishment of new laboratories across the region. Moreover, the availability of a cost-effective workforce provides Asia Pacific with a competitive advantage, thereby strengthening its role in the global central labs market.
Frequently Asked Questions on Central Lab
- What is a central lab?
A central lab is a centralized facility where laboratory tests and analyses are conducted for clinical trials. It provides standardized processes, equipment, and data management systems, ensuring accuracy, consistency, and regulatory compliance across multiple trial sites. - What services do central labs provide?
Central labs offer a wide range of services, including biomarker testing, genetic testing, safety testing, histopathology, and sample storage. These services support drug development by delivering high-quality data essential for clinical trial decision-making and regulatory submissions. - Why are central labs important in clinical trials?
Central labs are important because they minimize variability in test results, maintain consistency, and streamline trial operations. By centralizing laboratory testing, data integrity and comparability are enhanced, which is critical for reliable clinical research outcomes and regulatory approvals. - How do central labs ensure data quality?
Central labs ensure data quality by using standardized protocols, advanced instruments, and automated systems. They comply with Good Laboratory Practice (GLP) and international regulatory guidelines, which reduces errors and provides reliable datasets for clinical and scientific evaluation. - Who uses central lab services?
Central lab services are widely used by pharmaceutical companies, biotechnology firms, contract research organizations (CROs), and academic institutions. These stakeholders rely on central labs to provide consistent, high-quality testing to accelerate drug discovery, development, and clinical validation. - What is the central lab market?
The central lab market refers to the global industry providing centralized laboratory services for clinical trials. It encompasses specialized testing, logistics, and data management, supporting the growth of pharmaceutical and biotech research pipelines worldwide. - Which regions dominate the central lab market?
North America and Europe currently dominate the central lab market due to their advanced healthcare infrastructure, high research funding, and presence of major pharmaceutical companies. However, Asia-Pacific is witnessing the fastest growth, driven by rising clinical research activity.
Conclusion
The establishment of the Central Laboratory represents a transformative step in advancing diagnostics, research, and quality assurance. With cutting-edge infrastructure, skilled expertise, and adherence to global standards, it strengthens clinical decision-making and public health outcomes.
Market insights highlight steady growth, with North America leading while Asia-Pacific emerges as the fastest-growing region. Integration of automation, AI, and digital pathology enhances efficiency, despite challenges of high costs and complex trial management. Overall, the Central Lab serves as a cornerstone for innovation, collaboration, and excellence, positioning itself as a critical driver of progress in global healthcare and clinical research markets.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



