Table of Contents
Introduction
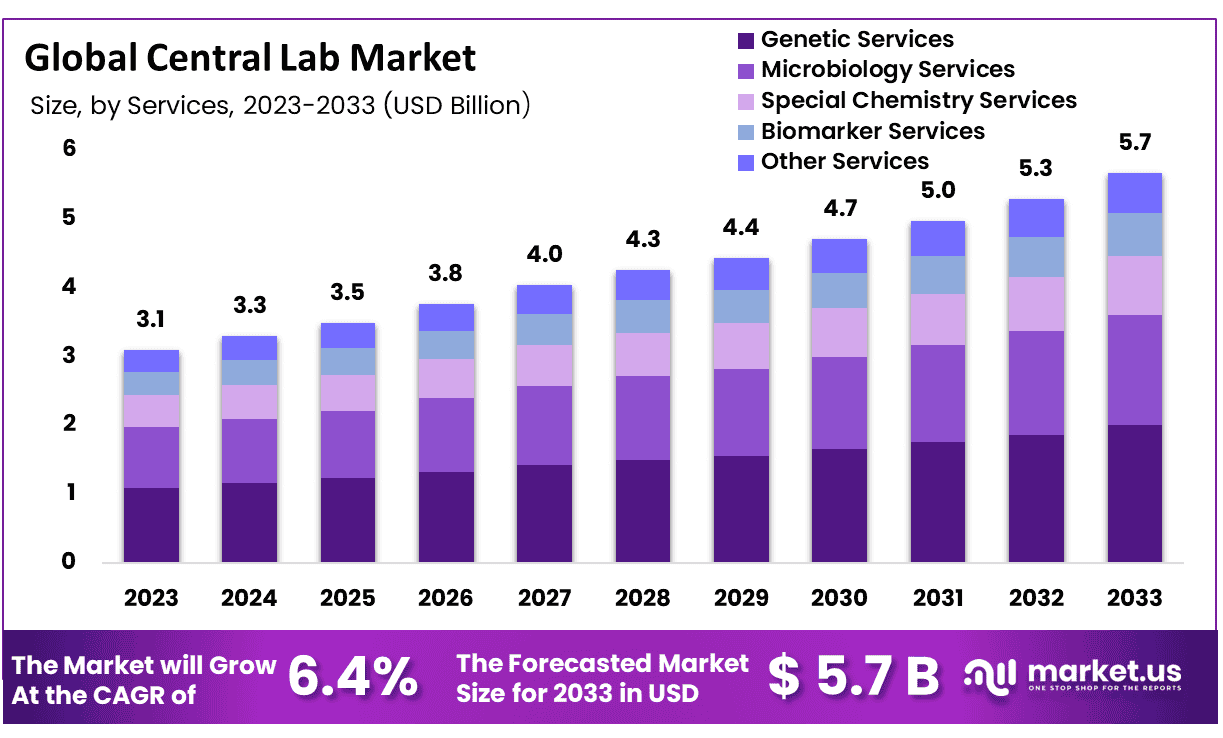
Global Central Lab Market size is expected to be worth around USD 5.7 Billion by 2033 from USD 3.1 Billion in 2023, growing at a CAGR of 6.4% during the forecast period from 2024 to 2033.
Central laboratories, essential in clinical trials, specialize in providing extensive and specialized testing services for pharmaceutical and biotech companies. These labs are instrumental in ensuring the standardization and quality of tests across different trial sites, thereby guaranteeing reliable data collection.
The growth of central labs is primarily driven by the increased investments in research and development (R&D) by the biopharmaceutical sector and a shift towards outsourcing laboratory services. This outsourcing helps reduce the overall expenses associated with research, as companies aim to streamline costs without compromising service quality or data integrity. A 2022 survey among biopharmaceutical companies highlighted a continuing trend towards outsourcing, which is expected to sustain as companies focus more on core trial activities rather than in-house lab operations.
With the rising incidence of various diseases, there is a growing need for new drugs and treatments, which further propels the demand for central lab services. These labs support the development and testing of new pharmaceuticals and are integral to genetic testing for various genetic disorders. The scale of clinical trials underscores the significant role of central labs; as of 2022, the US alone registered approximately 134,359 clinical trials, representing about 32% of the global total, with the remaining 52% conducted outside the US. This ongoing research activity is anticipated to drive further growth in the central lab market.
Key Takeaways
- Market Size: Global Central Lab Market size is expected to be worth around USD 5.7 Billion by 2033 from USD 3.1 Billion in 2023.
- Market Growth: The market growing at a CAGR of 6.4% during the forecast period from 2024 to 2033.
- Service Analysis: The Biomarker Services accounted 37.6% revenue share in 2023.
- End-User Analysis: Pharmaceutical companies’ segment dominated the market in 2023 with 45% market share.
- Regional Analysis: In 2023, the North American region accounted for 38.7% and holding a USD 1.2 Billion value for the global central labs market.
- Standardization and Quality: These labs ensure high-quality, standardized testing across various clinical trial sites, which is crucial for consistent and reliable data collection.
- Demand for New Drugs: Rising disease prevalence has escalated the need for new drug development, further boosting the demand for central lab services.
- Clinical Trials: Central labs facilitate the global expansion of clinical trials, with a significant number of studies registered both in the US and internationally.
Central Lab Statistics
- Over 10 million clinical lab tests are performed annually at major institutions like the Cleveland Clinic.
- Clinical labs implement over 250 distinct products, standards, and guidelines to ensure testing quality.
- The CLSI has over 24,000 members with access to various lab standards.
- These labs are present in more than 75 countries worldwide.
- The standards set by such organizations impact the accuracy of millions of diagnostics tests across the globe.
- Lab accreditation programs have specific measures for over 15 different health areas including cardiac care, emergency departments, and perinatal care.
- The Joint Commission’s laboratory standards are a benchmark for over 22,000 U.S. health care organizations and programs.
- Annual accreditation involves rigorous oversight of operational and testing procedures to ensure patient safety and quality care outcomes.
- Labs accredited by the Joint Commission must adhere to over 250 standards for health and safety.
- Certification through ASCP includes a detailed credentialing process that validates professional competency in laboratory skills.
- ASCP credentials have been issued to over 635,000 lab professionals globally since 1928.
- Each year, ASCP awards approximately 780,000 continuing medical education credits to lab professionals.
- Clinical laboratory scientists perform vital tests for detecting conditions like leukemia and heart attacks.
- Molecular diagnostics within these labs support the detection of genetic disorders, infectious diseases, and cancers.
- ASCP’s certification exams are instrumental for maintaining high competency standards in pathology and lab medicine.
Central Lab Services Analysis
- Genetic Services Central laboratories are integral to advancing personalized medicine by offering a robust suite of genetic testing services. These include comprehensive genomic profiling, gene expression analysis, and next-generation sequencing. This range enables precise disease diagnosis and the customization of therapies, which are pivotal in clinical trials and drug development. The ability to deliver accurate, actionable genomic data is a cornerstone for developing targeted treatments and enhancing patient outcomes.
- Microbiology Services In the realm of microbiology, central labs provide essential services that include the detection of infectious diseases and antimicrobial susceptibility testing. Their advanced, high-throughput systems ensure rapid and accurate pathogen detection, which is crucial for both diagnostic purposes and the management of infectious diseases in clinical settings. This capability supports healthcare providers by offering timely and reliable data for treatment decisions.
- Special Chemistry Services Central labs offer specialized chemistry services that play a critical role in understanding metabolic processes and disease mechanisms. These services, including hormone assays and enzyme evaluations, are vital for the discovery and development of new therapies. They provide essential insights that help in tailoring treatments to individual metabolic profiles, thereby increasing the efficacy of therapeutic interventions.
- Biomarker Services Biomarker services in central laboratories are fundamental to the drug development process. They help in the identification and validation of markers that can predict disease progression, response to treatment, and patient outcomes. These services are seamlessly integrated with clinical trials, enhancing the capability to monitor efficacy and safety, thus accelerating the pipeline from research to market.
- Other Services Additionally, central labs provide a range of other services that support the end-to-end needs of drug development and clinical trials. These include bioanalysis, flow cytometry, and support for cell and gene therapies. Each service is designed to meet the stringent demands of modern medical research, ensuring that all phases of trial and development are supported by reliable and comprehensive data analysis.
Emerging Trends
- Integration of Robotics: Robotics are increasingly integrated into central labs for tasks like sample collection and processing, which enhances efficiency and reduces human error.
- Space Medicine: With programs like Artemis aiming for extended space travel, central labs are focusing on space medicine to study the impacts of space on human health.
- Material Science: Central labs are also developing materials that withstand extreme conditions, which has applications in aerospace and other industries.
- Information Technology: The massive data from space missions require advancements in IT to process and analyze data effectively.
- Global Health Monitoring: Increased global travel is contributing to the spread of infectious diseases, prompting central labs to focus on global health monitoring and safety measures for travelers.
- Digital Inclusion: High Altitude Platform Stations (HAPS) are expected to bring internet access to billions, aiding in education and healthcare access in remote areas.
- Integrated Sensing and Communication: New technologies that combine data collection and transmission are being developed, enhancing monitoring capabilities in environmental and urban planning.
- AI in Research: Artificial intelligence is being leveraged in labs to accelerate discoveries in fields such as genetics and pharmacology.
- Synthetic Data: To address privacy concerns, labs use synthetic data which mimics real data patterns without compromising individual privacy.
- Climate Solutions: Labs are also focusing on technologies that address climate change, including sustainable practices and environmental monitoring.
Use Cases
- Disease Research and Diagnostics: Central labs conduct crucial research and diagnostic tests across various diseases, enhancing our understanding and management of health conditions.
- Genomic and Proteomic Research: These labs utilize high-throughput technologies to perform genomic and proteomic analyses, crucial for advancing personalized medicine.
- Drug Development: Central labs play a vital role in the drug development process, from initial discovery through clinical trials.
- Environmental Monitoring: Labs are equipped to monitor environmental changes and assess the impact of pollutants on public health and safety.
- Biosecurity and Public Health Surveillance: They are central to efforts in monitoring and responding to infectious disease outbreaks or bioterrorism activities.
- Advanced Material Development: Research into new materials that can withstand extreme environments is conducted at central labs, often with applications in aerospace and other industries.
- Energy Efficiency Research: Central labs investigate high-efficiency power systems, renewable energy technologies, and energy conservation methods.
- Educational and Training Programs: Many central labs offer internships and fellowships, providing essential training and research opportunities for students and professionals.
- Agricultural and Food Safety Research: These labs conduct research to improve crop yields, resist pests and diseases, and ensure food safety.
- Nuclear and Particle Physics Research: Facilities like the High Flux Isotope Reactor and the Spallation Neutron Source provide unique capabilities for studying fundamental particles and forces.
Conclusion
Central laboratories are crucial to the advancement of medical science, particularly within the pharmaceutical and biotech sectors. Their pivotal role in ensuring the standardization and quality of clinical trials underpins reliable data collection, which is vital for drug development and disease research. As the demand for innovative healthcare solutions escalates, the reliance on central labs is expected to grow, driven by an increase in R&D investments and a strategic shift towards outsourcing laboratory services. This progression is set to expand the global market significantly, reinforcing central labs’ integral contribution to modern medicine and public health.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



