Table of Contents
Overview
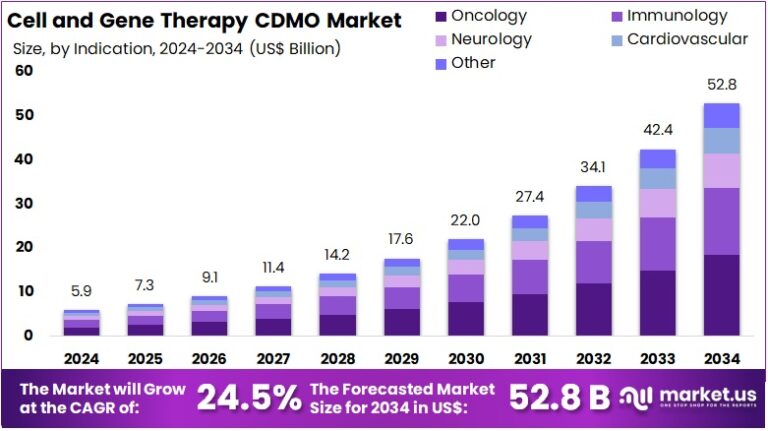
New York, NY – Aug 06, 2025 : The Global Cell and Gene Therapy CDMO Market is set to grow significantly. It is expected to reach around US$ 52.8 Billion by 2034, rising from US$ 5.9 Billion in 2024. This reflects a strong CAGR of 24.5% during 2025–2034. North America held a leading market position in 2024, with a 43.2% share and US$ 2.5 Billion in value. This growth is mainly driven by increasing demand for outsourced manufacturing and the rising number of therapy approvals. CDMOs are becoming vital players in this fast-growing healthcare sector.
A major driver for the CDMO market is the rise in cell and gene therapy clinical trials. The World Health Organization’s ICTRP data shows a steady global increase in such trials. In the U.S., the FDA had approved 19 gene therapy products by June 2024, compared to just one in 2017. This surge highlights a maturing and active therapy pipeline. CDMOs are helping meet this demand by offering specialized manufacturing support to biotech and pharmaceutical companies advancing these therapies.
Technological advancements are playing a key role in boosting CDMO growth. Modern manufacturing tools are helping companies improve efficiency and scalability. Automated processes and GMP-compliant systems are now standard in the industry. These innovations have helped reduce costs and increased global access to therapies. The World Health Organization continues to emphasize the need for strict manufacturing guidelines for biological products. CDMOs that invest in new technology are better positioned to meet rising client needs and expand their service offerings globally.
Supportive regulations are also fueling CDMO market growth. In the U.S., the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation has fast-tracked many innovative products. As of March 2025, several therapies have benefited from this program. Similarly, the European Medicines Agency (EMA) has issued clear guidelines for advanced therapy medicinal products (ATMPs). These regulatory frameworks encourage faster approvals and ensure patient safety. As a result, CDMOs are witnessing increased demand for regulatory-compliant manufacturing services across major markets like North America and Europe.
The focus on rare diseases is further accelerating the CDMO sector. About 300 million people worldwide live with rare conditions, according to the WHO. Gene therapies offer new treatment options for these patients. Products like Zolgensma and Elevidys have already received FDA approval, setting strong market precedents. Their success has encouraged further research and investment in rare disease therapies. CDMOs are stepping up by building capabilities tailored to these complex therapies. This trend is expected to drive innovation and improve treatment access for underserved patient groups.
Key Takeaways
- According to industry observers, the Cell and Gene Therapy CDMO market is projected to grow from US$ 5.9 Billion in 2024 to US$ 52.8 Billion by 2034.
- Experts highlight that this impressive growth represents a robust CAGR of 24.5% from 2025 to 2034, fueled by demand for next-gen therapeutics.
- Analysts note that Cell Therapy led the product category in 2024, capturing over 45.8% of the total market share within the CDMO sector.
- It’s been observed that oncology dominated application areas in 2024, contributing more than 45.8% to the total Cell and Gene Therapy CDMO demand.
- Market watchers confirm that North America was the leading region in 2024, holding over 43.2% share and generating US$ 2.5 Billion in revenue.
Regional Analysis
In 2024, North America dominated the global cell and gene therapy CDMO market, capturing over 43.2% share with a value of US$ 2.5 billion. This lead is driven by a robust biopharmaceutical ecosystem and strong regulatory support. The U.S. leads regional growth due to FDA’s fast-track approvals and clinical trial pathways. Government funding for rare diseases and novel therapies adds momentum. Biotech firms increasingly outsource to CDMOs for faster scale-up and compliance. This trend is rising, especially in therapies needing high precision and technical expertise.
North American CDMOs benefit from strong partnerships with academic institutions and research hospitals. These collaborations help advance early-stage drug development and GMP-compliant manufacturing. Innovation hubs and incubators support continuous progress across the region. As demand grows, many CDMOs are expanding or upgrading facilities to support next-generation therapies. Canada also plays a growing role, offering tax credits, grants, and public investments. Its emerging biotech scene attracts global contracts. This diversity strengthens North America’s leadership in the CDMO market.
Segmentation Analysis
In 2024, Cell Therapy led the product segment of the Cell and Gene Therapy CDMO Market with over 45.8% share. This dominance was due to the rising number of cell therapy programs in clinical trials. There was growing demand for both autologous and allogeneic treatments, especially in oncology and rare diseases. CDMOs with GMP-grade processing capabilities gained importance. Their role in expansion, cryopreservation, and regulatory compliance made them valuable partners. As a result, therapy developers increasingly relied on CDMOs to support the production and delivery of these advanced treatments.
Gene Therapy was the second-largest product segment, driven by clinical trials in genetic and neuromuscular disorders. The need for viral vectors like AAV and lentivirus posed technical challenges. Many therapy sponsors turned to CDMOs for help with compliant and scalable production. Gene-Modified Cell Therapy showed the fastest growth, fueled by CAR-T success in blood cancers. Developers sought CDMOs offering integrated services, from gene editing to final formulation. Growing investments and easier regulatory pathways further boosted outsourcing demand in this complex segment.
Key Players Analysis
Leading players are driving growth in the Cell and Gene Therapy CDMO market by offering end-to-end services. Charles River Laboratories expanded through its acquisition of Cognate BioServices, boosting viral vector and safety testing capabilities. Curia (formerly AMRI) is focusing on modular manufacturing and gene therapy scale-up. It is investing in facilities across North America and Europe. These companies provide integrated platforms from preclinical to commercial stages. Their services help therapy developers reduce timelines, streamline production, and bring advanced therapies to market more efficiently.
Emergent BioSolutions supports gene therapy production with expertise in biologics and biodefense. Its Baltimore site is key for viral vector manufacturing and GMP-compliant fill-finish services. Eurofins Scientific adds value through advanced bioanalytical testing and regulatory support. Its global reach supports gene-modified cell therapy development across regions. FUJIFILM Diosynth Biotechnologies is investing in high-capacity viral vector production in the U.S. and U.K. Major players like Thermo Fisher, Lonza, Catalent, and Samsung Biologics are also scaling capacity and forming strategic partnerships to accelerate innovation.
Emerging Trends
- Rising Demand for End-to-End Manufacturing Services: Biotech companies now prefer CDMOs that handle the full process from research to commercial production. Having everything in one place makes it easier to manage timelines and costs. This all-in-one approach reduces the need for switching vendors or repeating steps. It also lowers the risk of errors or delays in transferring materials. As a result, end-to-end CDMO partners are becoming more popular, especially for companies launching complex therapies. This trend is helping streamline drug development and speed up market entry.
- Growing Investment in Viral Vector Manufacturing: Gene therapies rely on viral vectors to deliver genetic material into cells. Because of this, CDMOs are expanding their capacity to produce these critical tools. They’re building new labs, adopting advanced bioprocessing systems, and hiring skilled talent. The goal is to meet the growing demand from both clinical trials and commercial-scale production. Improved viral vector supply ensures that gene therapy projects don’t face delays. This focus is also helping CDMOs stand out as key partners for gene therapy innovators.
- Shift Toward Modular and Flexible Facilities: Speed and adaptability are crucial in the biotech space. To stay ahead, CDMOs are designing modular and flexible production units. These setups can quickly switch between different therapy types, such as CAR-T cells or other cell-based treatments. This is ideal for personalized medicine, where small, custom batches are common. Modular facilities also allow faster upgrades and easier scaling. As therapies evolve, CDMOs with flexible systems can respond more efficiently to client needs.
- Surge in Partnerships and Mergers: Biotech companies are forming more partnerships with CDMOs to speed up development. At the same time, CDMOs are merging or acquiring other firms to expand services and reach. These collaborations bring in new technologies and global capabilities. They also help share knowledge and reduce time to market. As a result, the industry is becoming more connected. Companies that work together are better positioned to handle complex therapies and regulatory challenges.
- Embracing Automation and Digital Tools: CDMOs are increasingly turning to automation, AI, and digital systems to improve production. These tools help ensure high-quality output, reduce human error, and shorten timelines. For example, smart manufacturing platforms can monitor conditions in real time and adjust processes automatically. AI and data analytics also support better decision-making. By using digital tech, CDMOs can offer more efficient, safe, and consistent services—critical for sensitive treatments like cell and gene therapies.
- Stronger Focus on Regulatory Compliance: Cell and gene therapies must meet strict global safety standards. CDMOs are investing more in regulatory know-how and quality systems. This includes training, digital documentation, and cleanroom operations. By staying compliant, they help clients avoid costly delays or product recalls. Having strong regulatory support is especially important for startups or small biotech firms that may lack internal expertise. CDMOs that offer regulatory guidance are becoming trusted long-term partners.
- Expansion into Emerging Regions: Demand for advanced therapies is rising in areas like Asia-Pacific and Latin America. CDMOs are expanding into these regions to tap new markets and get closer to patients. These expansions bring localized services, lower production costs, and access to growing talent pools. They also help biotech firms overcome logistical and regulatory barriers. By entering emerging markets, CDMOs can support a wider range of companies while improving global healthcare access.
Use Cases
- Manufacturing Personalized Cancer Treatments: CDMOs play a key role in making personalized cancer therapies like CAR-T. These treatments use a patient’s own immune cells. The cells are collected, modified in a lab, and then infused back into the patient to fight cancer. This process must follow strict quality and safety rules. CDMOs have the tools and expertise to make these therapies quickly and safely. They also help reduce delays by streamlining the manufacturing process. Personalized treatments are often time-sensitive, so reliable CDMO support is essential. By managing production, CDMOs help healthcare providers deliver advanced care faster. This improves patient outcomes and makes these complex therapies more accessible.
- Production of Gene Therapies for Rare Diseases: CDMOs help create gene therapies that treat rare and inherited diseases. These disorders often have no cure or limited treatment options. Gene therapy works by replacing or fixing the faulty gene that causes the illness. CDMOs support this by designing and producing custom gene vectors. They also ensure the final product is safe and effective. Their teams follow strict regulations and use advanced technology. Many small biotech firms rely on CDMOs because they lack large-scale facilities. With CDMO support, companies can focus on discovery while experts handle production. This brings hope to patients with rare, hard-to-treat conditions.
- Scaling Up Clinical Trial Materials: During clinical trials, drug developers need high-quality therapies in small batches. CDMOs specialize in making these clinical trial materials. They ensure every batch meets the safety and quality standards needed for human testing. This includes aseptic processing, GMP compliance, and batch documentation. Scaling production for trials requires precision, flexibility, and expertise. CDMOs provide all of these. They also adapt quickly if trial requirements change. Their support helps biotech firms avoid delays in clinical studies. This speeds up the drug development process. In short, CDMOs are key partners in the early phases of therapy development and testing.
- Cryopreservation and Logistics Support: Many cell therapies must be frozen to stay viable during storage and transport. CDMOs provide specialized cryopreservation services to meet this need. They use advanced freezing techniques and validated equipment. These ensure that cells maintain their potency and safety until they reach the patient. CDMOs also manage cold-chain logistics. This includes real-time monitoring and temperature-controlled shipping. Safe delivery is crucial because damaged cells can’t be used. CDMOs handle these steps with precision, reducing risks for patients and providers. Their expertise supports smooth delivery from the lab to the clinic. This makes cell-based therapies more reliable and accessible.
- Process Development and Optimization: CDMOs help biotech companies develop better ways to make therapies. This is called process development. It involves refining each step of the manufacturing workflow. The goal is to improve quality, reduce costs, and scale up efficiently. CDMOs test different methods and select the best ones for large-scale use. They also ensure the process meets industry standards and regulatory rules. Optimizing manufacturing early helps avoid problems later. It also speeds up time to market. By working with CDMOs, biotech firms can focus on innovation while experts handle the complex manufacturing process. This collaboration ensures smooth, scalable production.
- Quality Testing and Regulatory Support: Safety and compliance are critical in cell and gene therapy. CDMOs offer full support for quality testing. They check every batch for purity, potency, and contamination. This ensures treatments are safe for patients. CDMOs also help with documentation required by regulatory agencies like the FDA and EMA. This includes creating reports, following GMP standards, and preparing for audits. Their teams understand the complex rules for advanced therapies. They guide biotech firms through each regulatory step. This helps reduce approval delays and improves product safety. With CDMO support, companies can confidently move therapies through the pipeline.
- Technology Transfer from Lab to Commercial Scale: When a therapy shows success in the lab, it needs to be produced at scale. CDMOs help transfer this technology from research to full-scale manufacturing. This is known as tech transfer. It involves translating lab-based processes into industrial workflows. CDMOs document each step, identify risks, and ensure consistency. They also help validate the process for regulatory approval. This ensures quality remains high even when production volumes increase. A smooth tech transfer is vital for launching therapies on time. With experienced CDMO partners, biotech firms can scale up without major disruptions. This supports faster access to life-saving treatments.
Conclusion
In conclusion, the Cell and Gene Therapy CDMO market is growing rapidly due to rising demand for advanced therapies, strong regulatory support, and ongoing innovation. CDMOs are playing a key role by offering flexible, end-to-end solutions for therapy development, manufacturing, and compliance. Their expertise is helping biotech firms overcome technical challenges and bring treatments to patients faster.
As more cell and gene therapies enter the pipeline, the need for reliable CDMO partners will continue to rise. With investments in technology, global expansion, and strategic partnerships, CDMOs are well-positioned to support future growth. This market will remain vital for advancing next-generation healthcare solutions across the world.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



