Table of Contents
Introduction
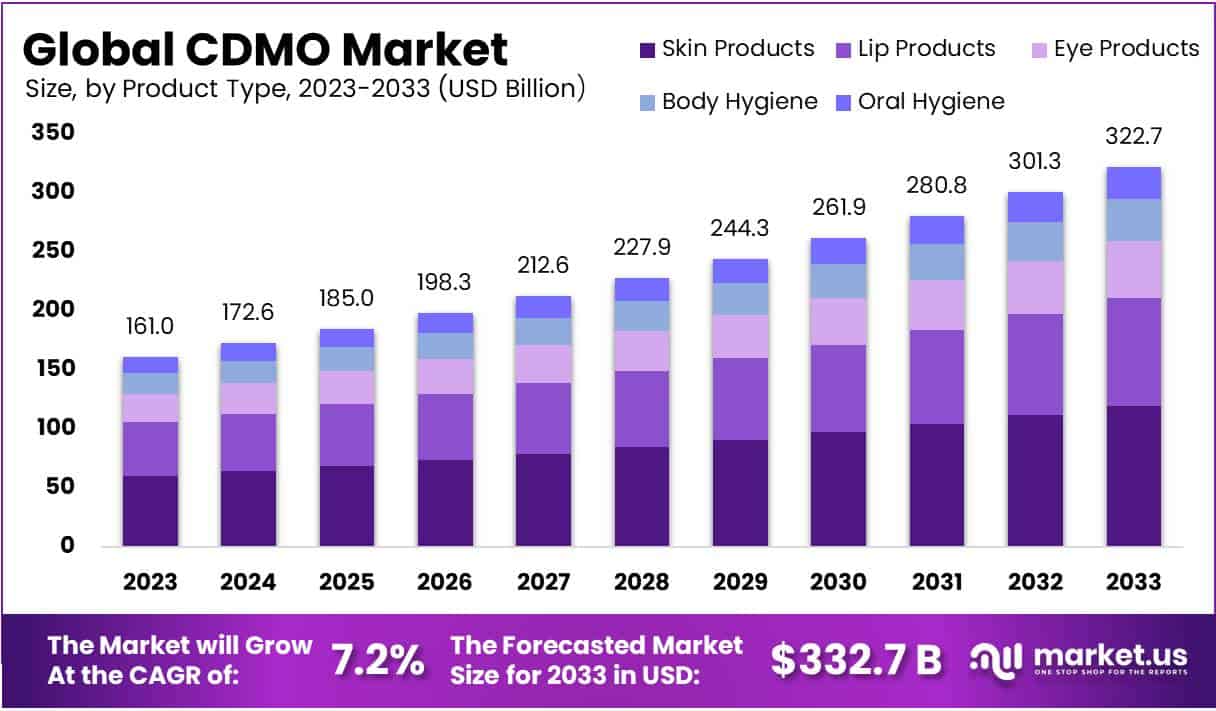
Global CDMO Market Size is expected to be worth around USD 322.7 Billion by 2033 from USD 161 Billion in 2023, growing at a CAGR of 7.2% during the forecast period from 2024 to 2033. In 2023, North America led the market, achieving over 35.1% share with a revenue of US$ 56.5 Billion.
The market’s growth is driven by expansions, acquisitions, and investments in advanced manufacturing. Companies like Fujifilm Diosynth Biotechnologies and Lonza are heavily investing in biomanufacturing to meet rising demand for biologics and advanced therapy medicinal products (ATMPs). Additionally, companies such as Bachem and WuXi STA are boosting production capacities for complex molecules like peptides and oligonucleotides to cater to specialized needs.
Strategic acquisitions are shaping the industry. For instance, Ajinomoto acquired Forge Biologics to strengthen its biologics capabilities. However, challenges like market uncertainties, logistics issues, and increasing costs remain. To tackle these, companies are forming collaborations, such as Lonza’s partnership with Evotec in December 2023, which focuses on advancing personalized medicine by combining CDMO services and drug discovery expertise.
Recent developments emphasize capacity expansion. Thermo Fisher Scientific launched a GMP-certified facility in the Netherlands in January 2024 for cell and gene therapy trials in Europe. Catalent’s October 2023 acquisition of Metrics Contract Services improved its clinical packaging for biologics. Samsung Biologics and Boehringer Ingelheim’s joint venture in September 2023 aims to increase biopharmaceutical production. These steps enhance capabilities and help companies meet the growing complexity of drug development and manufacturing.
Key Takeaways
- Market Forecast: The CDMO market is predicted to reach USD 322.7 billion by 2033, expanding at a 7.2% CAGR from 2024 to 2033.
- Product Leadership: Skin care products hold a 37.1% market share in 2023, significantly influencing market growth and innovations.
- CMO Dominance: APIs lead in the CMO sector with a 41.5% market share, indicating robust manufacturing growth.
- Clinical Trials Leadership: Clinical trial services hold a dominant 47.2% market share in 2023, fueled by strategic partnerships and innovations.
- Growth Drivers: The CDMO market growth is spurred by Western lifestyles, economic advancement, and population growth.
- Expansion Challenges: Market expansion is restricted by stringent government regulations and fewer approvals for biologics and small molecules.
- Industry Opportunities: Increasing partnerships with CDMOs are in response to the rising demand for innovative therapeutics and expanded service capabilities.
- Trend Impact: Research and development funding, especially in the U.S., plays a significant role in driving the CDMO market’s growth.
- Regional Dynamics: North America leads with a 35.1% market share in 2023, while the Asia Pacific region records the highest CAGR due to lower costs and increased chronic disorders.
CDMO Market Statistics
- Baxter sold its CDMO business for $4.25 billion in October 2023, forming Simtra BioPharma Solutions.
- Samsung Biologics is investing over $1.46 billion in a biomanufacturing plant with a capacity of 180,000 liters, expected to be operational by 2025.
- Lotte Biologics announced a $3 billion investment to build three biomanufacturing plants in South Korea with a combined capacity of 360,000 liters.
- Fujifilm Diosynth Biotechnologies is investing $2 billion in North Carolina to add manufacturing suites with 4 x 20,000-L bioreactors each.
- Fujifilm plans an additional $1.6 billion investment for expansions in Denmark and Texas, including 8 x 20,000-L bioreactors in Denmark.
- Lonza is allocating $935 million for two new mammalian drug-substance manufacturing facilities in Switzerland and New Hampshire.
- Cambrex sold its Drug Product Business Unit to Noramco in November 2023, after acquiring it for $425 million in 2018.
- Bachem is investing over $1.4 billion to expand peptide and oligonucleotide manufacturing in Switzerland and globally.
- Agilent Technologies launched a $725 million expansion to double nucleic acid manufacturing in Colorado by 2026.
- WuXi STA plans to increase its solid-phase peptide synthesizer capacity to exceed 20,000 liters.
- Ajinomoto is acquiring Forge Biologics for $554 million, with the deal expected to close in December 2023.
- Bain Capital Private Equity acquired Fabbrica Italiana Sintetici (F.I.S.) for approximately $781 million in July 2023.
- CordenPharma is expanding oligonucleotide manufacturing in Colorado through a multi-phase investment starting in 2023.
- Ascend Gene & Cell Therapies raised $132.5 million in 2023 to focus on AAV vector production.
- Private equity investments in the European healthcare sector decreased by 27% in 2022.
- The top five CDMO players hold only 15% of the total market share.
- Most CDMO companies generate less than $50 million in annual revenue.
Emerging Trends in the CDMO Sector
- Adoption of Advanced Therapeutics and Flexibility: The rising demand for advanced therapeutics, such as cell and gene therapies, is reshaping CDMO operations. To address this demand, CDMOs are adopting flexible manufacturing strategies, including modular facilities. These facilities enable quick adaptation to different product types and production scales, accelerating the transition from development to market readiness.
- Emphasis on Digital Transformation: Digitalization is transforming the CDMO sector by improving efficiency and data management. Advanced digital tools and automation technologies are streamlining operations, enhancing decision-making, and fostering stronger collaborations with clients. These innovations ensure optimized production processes and create a more dynamic and integrated workflow.
- Focus on Sustainability Initiatives: Environmental sustainability is becoming a priority for CDMOs. Organizations are implementing eco-friendly practices and developing sustainable packaging solutions to reduce their environmental impact. These efforts align with stricter regulatory standards and position CDMOs as responsible partners in the biopharmaceutical industry.
- Customer-Centric Approaches: CDMOs are shifting towards customer-focused models by closely engaging with biopharma clients to understand their specific needs. Customized services, combined with innovation driven by client feedback, enhance customer satisfaction. This approach ensures efficient operations tailored to precise client requirements.
Use Cases of CDMO Services
- Accelerating Time to Market: CDMOs enable faster delivery of pharmaceuticals to the market, which is critical for time-sensitive treatments. Flexible manufacturing ensures new drugs reach patients promptly, improving outcomes and maximizing commercial success.
- Expertise in Complex Pharmaceuticals: Specializing in products like biologics and sterile injectables, CDMOs manage complex production processes, ensuring regulatory compliance and high-quality standards. This expertise is crucial for precision-driven pharmaceutical manufacturing.
- Enhancing Capacity Management: During periods of high demand, such as the COVID-19 pandemic, CDMOs expand manufacturing capacity to prevent drug shortages. This flexibility ensures a steady supply of essential medications, supporting healthcare systems in critical times.
- Fostering Innovation through Partnerships: Collaborating with biotech startups, CDMOs provide the resources and expertise needed to develop new therapies. These partnerships accelerate the creation of innovative treatments, especially for smaller companies without in-house manufacturing capabilities.
- Stabilizing the Global Supply Chain: CDMOs enhance the stability of the pharmaceutical supply chain by diversifying production sites and improving logistics. Their role is vital during trade restrictions or disruptions, ensuring a consistent supply of essential medicines worldwide.
Conclusion
The CDMO market is experiencing significant growth, driven by investments, acquisitions, and capacity expansions to meet rising demand for biologics and advanced therapies. Companies are focusing on advanced manufacturing, digitalization, and sustainability to enhance efficiency and meet regulatory standards. Key trends include strategic collaborations, flexible production, and customer-centric models.
Challenges like high costs and regulatory hurdles persist but are mitigated through partnerships and innovative strategies. The market, valued at $322.7 billion by 2033, is shaped by regional dynamics, with North America leading while Asia Pacific grows rapidly. CDMOs play a critical role in accelerating drug development and ensuring supply chain stability.


