Table of Contents
Introduction
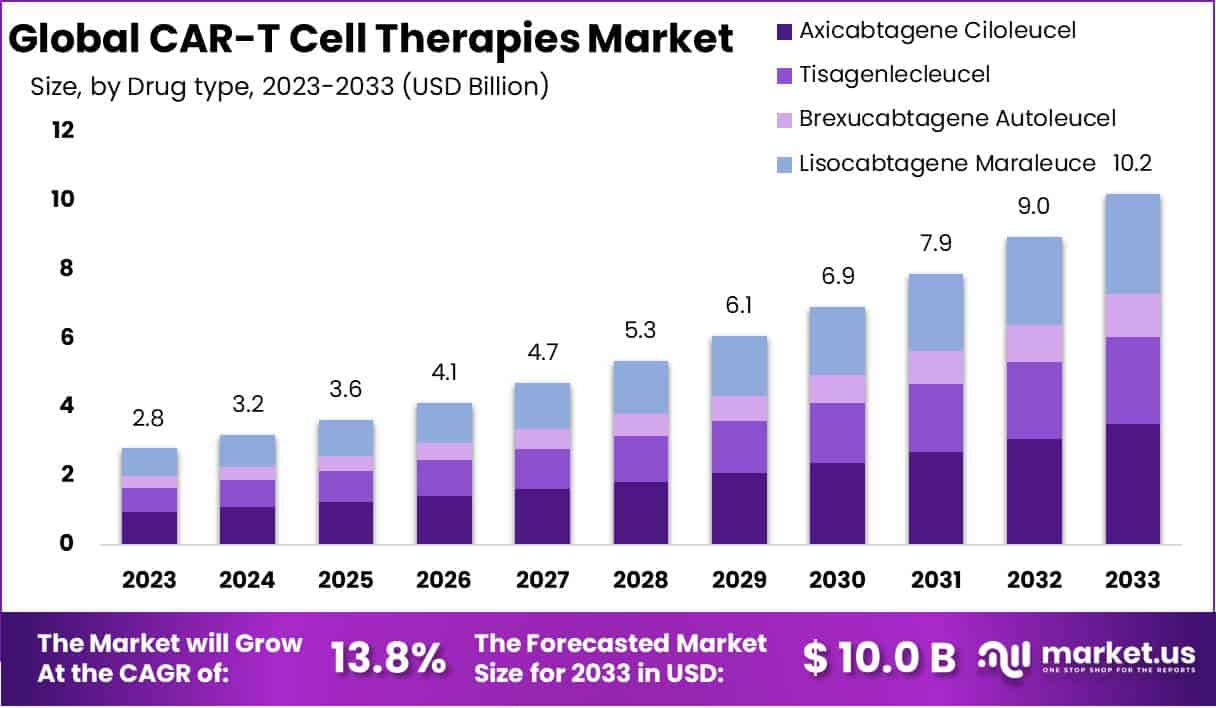
The global CAR-T cell therapies market is projected to grow significantly, with its valuation expected to increase from USD 2.8 billion in 2023 to USD 10.2 billion by 2033, at a compound annual growth rate (CAGR) of 13.8%. This surge is attributed to the increasing prevalence of targetable cancers such as leukemia and lymphoma, and the potential expansion into treating solid tumors like breast and lung cancer. As clinical applications evolve, regulatory approvals in North America and Europe, along with expansion in Asia, signify a global acceptance of this groundbreaking treatment.
Innovations in CAR-T therapy are transitioning from second to fourth generation, enhancing treatment efficacy, safety, and targeting capabilities. The growth of this market is further propelled by the rising demand for personalized medicine, exemplified by these therapies’ approach of modifying a patient’s T-cells to combat cancer. However, challenges such as high costs, limited accessibility, and severe side effects like cytokine release syndrome persist, driving the need for innovative reimbursement models and improved treatment profiles to expand patient access.
Recent industry movements underscore the market’s dynamic nature. In October 2023, Gilead Sciences expanded its oncology footprint by acquiring Kite Pharma for $5.4 billion, gaining the pioneering CAR-T therapy, Yescarta. September 2023 witnessed Pfizer’s $3 billion collaboration with Allogene Therapeutics to develop off-the-shelf CAR-T therapies, potentially revolutionizing cancer treatment accessibility. Additionally, Merck entered the CAR-T arena in August 2023 with the launch of Abcema for treating multiple myeloma, highlighting the therapy’s broadening applications.
Strategic collaborations continue to drive innovation in the sector. Bluebird Bio’s recent success in a Phase 2 trial for pediatric acute lymphoblastic leukemia emphasizes the potential of CAR-T therapies in pediatric oncology, achieving high remission rates and durable responses. These advancements not only highlight the rapid progress in treatment options but also the industry’s commitment to enhancing efficacy and patient outcomes through ongoing research and strategic partnerships.
Overall, the CAR-T cell therapies market is on a trajectory of robust growth and innovation. Companies are not only enhancing existing therapies but are also actively exploring new combinations with other immunotherapies to improve efficacy and reduce relapse rates in cancer patients. With continuous research aimed at overcoming current limitations and expanding the therapy’s applications, the future of CAR-T cell therapies looks promising, making it a critical area for investment and development in the oncology sector.
Key Takeaways
- The market is projected to expand from USD 2.8 billion in 2023 to USD 10.2 billion by 2033, growing at a CAGR of 13.8% from 2024 to 2033.
- Axicabtagene ciloleucel leads the market with a 34.4% share in 2023, fueled by its FDA approval for treating large B-cell lymphoma.
- The research modality captures a 71.5% market share in 2023, indicative of significant participation from research institutions and numerous clinical developments.
- CD19/CD22 antigens are predominant, accounting for a 53.9% market share in 2023, reflecting their effectiveness in B-cell malignancy treatments.
- Lymphoma holds a 57.1% market share in 2023, supported by several FDA-approved CAR-T cell therapies targeting various lymphoma types.
- Hospitals are the leading end-users with a 62.8% market share in 2023, due to their advanced facilities and capabilities alongside higher patient admissions.
- North America significantly influences the market, contributing 61.49% with a valuation of USD 1.8 billion in 2023, propelled by a high chronic disease burden and strong R&D efforts.
CAR-T Cell Therapies Statistics
- Global Impact and Cost:
- Asia accounts for over 60% of the global population and 40% of the world’s GDP.
- The Asia-Pacific region has driven 70% of global economic growth in the past two decades.
- In 2022, approximately 1,432 CAR-T therapies were administered, with usage increasing annually.
- CAR-T Therapy Overview:
- CAR-T therapy is primarily approved after 1 or 2 lines of prior therapy; however, 88% of patients with Multiple Myeloma (MM) and 43% with Diffuse Large B-Cell Lymphoma (DLBCL) start treatment after ≥4 therapy lines.
- The price of each CAR-T cell product in China is about $175,000, while in Singapore, Kymriah and Yescarta are sold for around US$375,000 each.
- Approximately 342 clinical trials involving CAR-T cells had been conducted by 2021.
- Financial Aspects:
- Some commercial insurance systems in China cover about 50% of CAR-T cell therapy costs.
- In Japan, most funding for CAR-T therapy comes from public health insurance, with patients under 70 typically paying 30% of the total medical cost.
- The cost of CAR-T cells in the US is 40 to 200 times higher than the GDP per capita.
- Manufacturing and Clinical Outcomes:
- Automated, closed cell therapy manufacturing can reduce the required manufacturing headcount by over 70% per drug product.
- To produce 4,500 doses annually, a cell therapy manufacturer needs a facility of at least 200,000 sq ft, with initial costs exceeding $160 million for construction and equipment.
- Chemotherapy cures about 70% of patients overall, but 30% do not respond.
- The 5-year survival rate in the US stands at 56%.
- Recurrence rates after CAR-T cell therapy are 7% for low-risk patients, 27% for intermediate-risk, and 53% for high-risk patients.
- Clinical Trial Data:
- Cytokine release syndrome (CRS) occurs in 56.2% to 71.7% of CAR-T cell therapy recipients with hematologic malignancy.
- In the BELINDA trial, 61% of patients experienced CRS, with 5% experiencing severe cases.
- The TRANSFORM trial reported CRS in 49% of participants, with a severe event in only 1% of cases.
- Prevalence of Urinary System Tumors: Urinary system tumors account for about 24% of all new cancers in the United States.
- Biochemical Recurrence Rate for Prostate Cancer: Following radical treatment, prostate cancer has a biochemical recurrence rate of up to 50% within three years.
- CAR T Cells in RCC (Renal Cell Carcinoma):
- Incidence: Approximately 81,610 new cases expected in 2024.
- Mortality: About 14,390 deaths anticipated in 2024.
- CAR T Cells in Bladder Cancer (BCa):
- New Cases: Around 83,190 expected in 2024.
- Deaths: Approximately 16,840 anticipated in 2024.
- Five-year survival rate for non-metastatic BCa: Up to 90%.
- Five-year survival rate for metastatic BCa: Less than 15%.
- CAR T Cells in Prostate Cancer (PCa):
- New Cases: Estimated 300,000 in 2024.
- Deaths: Anticipated 35,250 in the same year.
- Five-year survival rate for localized PCa: 98%.
- Five-year survival rate for metastatic PCa: 30%.
- Overall Response Rates (ORR):
- Tisagenlecleucel (JULIET study): 53.0% ORR at a median follow-up of 40.3 months.
- Axicabtagene ciloleucel (ZUMA-1 study): 83% ORR, with a complete response (CR) rate of 58% at a median follow-up of 27.1 months.
- Lisocabtagene maraleucel (TRANSCEND study): 73% ORR, with a CR rate of 53% at a median follow-up of 18.8 months.
- Event-Free Survival (EFS):
- ZUMA-7 trial (axi-cel): Median EFS of 8.3 months compared to 2.0 months in the standard of care (SOC) group.
- TRANSFORM study (liso-cel): Median EFS of 10.1 months compared to 2.3 months in the SOC group.
- Incidence of Cytokine Release Syndrome (CRS) and Neurotoxicity:
- Axi-cel: Higher frequency of CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) but largely controllable.
- Tisa-cel and Liso-cel: Generally lower incidence of CRS and ICANS compared to axi-cel.
- Real-World Data (RWD):
- Tisa-cel: 61.8% ORR, with 6-month overall survival (OS) and progression-free survival (PFS) rates of 70.7% and 38.7%, respectively.
- Axi-cel: 82% ORR, with 12-month PFS and OS rates of 45% and 64%, respectively.
Emerging Trends
- Advancements in Gene-Transfer Technologies: The field of CAR-T cell therapies is witnessing significant advancements, particularly in gene-transfer technologies. Innovations such as transposon and CRISPR tools are enhancing the efficiency with which T-cells can be modified—a critical step in producing CAR-T therapies. These technological improvements not only expedite the testing of new modifications but also reduce the costs associated with developing next-generation treatments. As a result, these advancements are setting the stage for faster development of innovative CAR-T therapies, potentially transforming cancer treatment paradigms.
- Enhanced Durability and Functionality: Researchers are actively developing new strategies to improve the persistence and functionality of CAR-T cells. One notable innovation is the CAR-Enhancer (CAR-E) platform, which aims to boost the activity of CAR-T cells. This platform helps the cells retain a memory of cancer cells, enabling them to remain active for longer periods. By enhancing the durability of CAR-T cells, these strategies could significantly reduce relapse rates in patients, marking a crucial step forward in the treatment of cancer.
- Safer Delivery and Management of Side Effects: Managing the side effects of CAR-T cell therapies, such as cytokine release syndrome (CRS), remains a priority. To improve patient safety, researchers are developing diagnostic tests and employing predictive modeling techniques to identify severe cases of CRS early after infusion. These efforts are crucial in mitigating risks associated with CAR-T therapies and enhancing the overall treatment experience for patients.
- Outpatient Treatment Accessibility: There is a growing trend towards facilitating the accessibility of CAR-T therapies as outpatient treatments. This shift aims to lessen the reliance on extensive hospital infrastructure, potentially reducing treatment costs and broadening the availability of these therapies to a larger number of patients. By simplifying the delivery process, this trend not only makes CAR-T cell therapies more accessible but also supports a broader implementation across various healthcare settings.
Use Cases
- Revolutionizing Blood Cancer Treatment: CAR-T cell therapies represent a significant advancement in the treatment of blood cancers, including B-cell leukemias, lymphomas, and multiple myeloma. These innovative therapies are particularly beneficial for patients who have not seen success with conventional treatments or who have experienced a relapse. By harnessing the body’s immune system to target and destroy cancer cells, CAR-T therapies offer a promising alternative, changing the landscape of cancer treatment.
- Achieving Long-term Remission: One of the most compelling outcomes of CAR-T cell therapy is its ability to induce long-term remission. In cases of chronic lymphocytic leukemia, some patients have achieved remission lasting over a decade following a single infusion. This highlights the potential of CAR-T therapies not only as a treatment but as a possible long-term solution for certain cancers.
- Exploring Broader Applications: Initially developed for oncology, the use of CAR-T cell therapies is now expanding to other medical areas. Research is underway to explore the potential of these therapies in treating autoimmune diseases and other conditions that could benefit from precise, targeted immune responses. This exploration could open new avenues for CAR-T cell applications, potentially transforming the approach to treatment in various diseases.
Conclusion
The CAR-T cell therapies market is poised for impressive growth, driven by advances in cancer treatment and the rise of personalized medicine. As these therapies evolve, they are broadening their reach beyond leukemia and lymphoma to potentially address solid tumors. Despite facing challenges such as high costs and significant side effects, ongoing innovations and strategic partnerships are enhancing treatment effectiveness and accessibility. The market’s expansion is further supported by strong research and development, particularly in North America and Europe, setting a promising path forward. With continuous efforts to improve efficacy and manage side effects, CAR-T cell therapies are expected to transform oncology, offering new hope to patients and attracting considerable investment in the healthcare sector.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



