Table of Contents
Overview
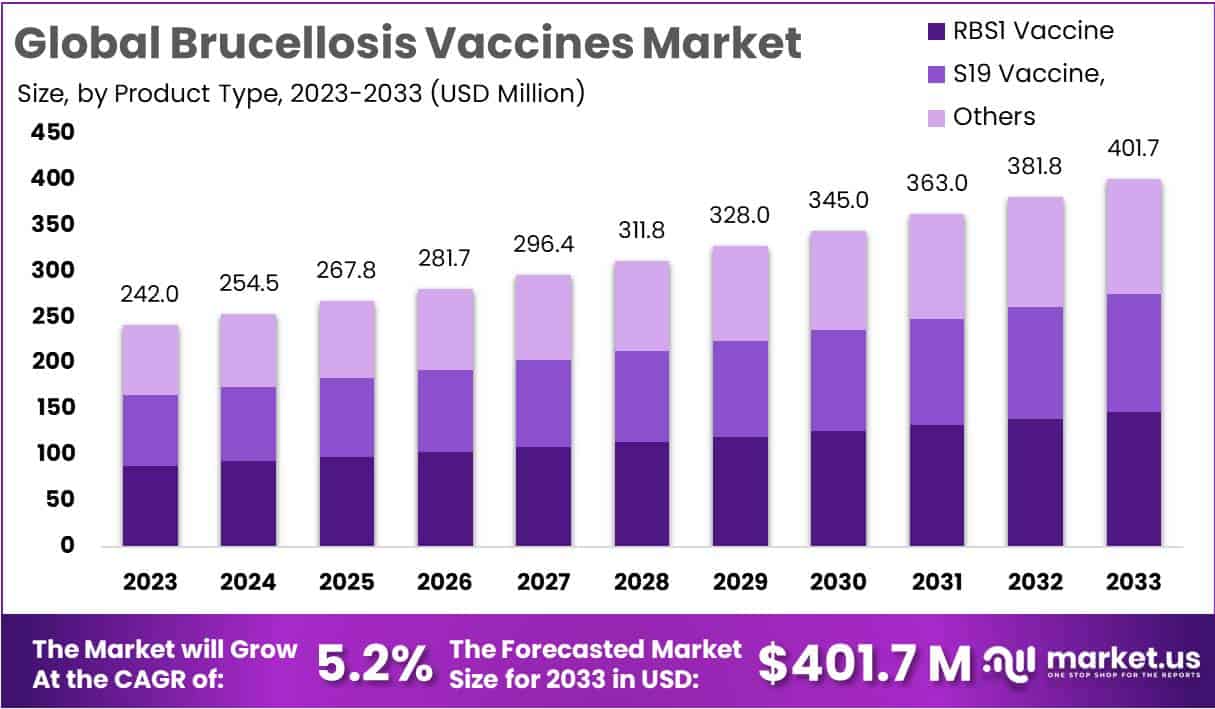
The Brucellosis Vaccines Market is projected to reach USD 401.7 million by 2033, rising from USD 242 million in 2023, with a compound annual growth rate (CAGR) of 5.2% during 2024–2033. The market growth is primarily driven by the continued high global disease burden. According to CDC’s Emerging Infectious Diseases (2023), approximately 2.1 million human brucellosis cases occur annually worldwide. This figure highlights the persistent transmission cycle between livestock and humans, emphasizing the critical role of vaccination in disease prevention and control.
Policy guidance from global health organizations reinforces the centrality of animal vaccination. The World Health Organization (WHO) advocates eliminating infection in livestock as the most effective means of preventing human brucellosis. Similarly, the World Organisation for Animal Health (WOAH) continues to recommend live-attenuated vaccines—such as B. abortus S19 and RB51 for cattle and B. melitensis Rev.1 for small ruminants. The alignment of national programs with these international standards sustains steady demand for these vaccines across endemic regions.
Moreover, economic analyses have reinforced vaccination as a cost-effective public health investment. The FAO’s One Health cost–benefit studies indicate that vaccination-based strategies result in positive net monetary gains by lowering animal losses and reducing human illness costs. Consequently, governments and donors are extending financial support for multi-year vaccination programs, ensuring stable procurement and delivery networks. This financial validation continues to strengthen national commitments to sustained disease-control efforts.
Finally, food-safety messaging and regulatory actions also influence market growth. Warnings against raw milk consumption and unpasteurized dairy products have increased public awareness of zoonotic risks. This rising consumer vigilance pushes producers to maintain herd immunity, leading to consistent vaccine usage among veterinary services and livestock operators in high-risk areas.
Regional Insights and Strategic Outlook
Regional surveillance reveals significant variation in disease incidence and vaccine adoption. In the European Union (EU/EEA), only 199 confirmed human brucellosis cases were reported in 2022, representing 0.04 per 100,000 population. This low rate demonstrates the success of systematic vaccination, testing, and culling programs. Conversely, Asia, the Middle East, and Africa continue to record higher infection rates, where national control strategies are expanding to emulate the EU’s eradication model. Consequently, vaccine demand remains concentrated in endemic regions, with rapid program scale-ups anticipated over the next decade.
In North America, government programs also underpin stable market performance. The U.S. Department of Agriculture’s APHIS continues to license and monitor field use of the RB51 vaccine for bovine brucellosis. Regulatory documentation emphasizes strict handling protocols for vaccinators and veterinary workers, further reinforcing structured purchasing and cold-chain infrastructure. These operational requirements sustain recurring procurement and training needs within the national veterinary system.
The One Health approach—integrating human, animal, and environmental health—remains central to long-term market stability. Global initiatives underscore that controlling brucellosis in animals reduces human infections, agricultural losses, and household poverty. Consequently, ministries of health and agriculture in endemic countries are aligning resources to expand immunization coverage, surveillance, and diagnostic capacity.
In summary, the brucellosis vaccines market is poised for stable and sustained growth. Expansion is driven by the persistent global burden, WHO and WOAH vaccination advocacy, favorable economic evidence, and regulatory backing for animal health programs. As more nations implement integrated control strategies, consistent vaccine procurement and usage are expected to continue through 2033, ensuring strong market performance and advancing global zoonotic disease control objectives.
Key Takeaways
- The Brucellosis Vaccines Market is projected to expand significantly, attaining an estimated valuation of approximately USD 401.7 million by 2033.
- In 2023, the market was valued at around USD 242 million, establishing a strong foundation for the anticipated growth over the next decade.
- The market is forecasted to grow steadily at a compound annual growth rate (CAGR) of 5.2% from 2024 to 2033.
- Among product types, the RBS1 Vaccine dominated with a 36.6% market share, indicating a strong preference among users and veterinarians.
- The S19 Vaccine followed as the next leading product, showcasing its continued relevance in Brucellosis prevention programs across several regions.
- DNA Vaccines held a commanding 32.4% market share, reflecting increasing confidence in their efficacy and advanced preventive capabilities.
- The Cattle Segment accounted for a substantial 56.5% share, emphasizing the focus on controlling Brucellosis infections in bovine livestock globally.
- Veterinary Hospitals and Clinics emerged as the dominant distribution channel, capturing 76.9% of the market due to their accessibility and reliability.
- North America led the global market with a 42.5% share, supported by high disease prevalence and advanced veterinary infrastructure.
- Growth is driven by factors such as increasing livestock populations, supportive government initiatives, and technological advancements in vaccine development.
- The market faces restraints including limited vaccine accessibility in remote areas, high production costs, and safety-related concerns.
- Promising opportunities exist in emerging and underserved markets, collaborations, and increased R&D investments for improved vaccine innovation.
- Key trends include a rising focus on combination vaccines, the One Health approach, and greater adoption of DNA-based vaccine technologies.
Regional Analysis
In 2023, North America dominated the Brucellosis Vaccines Market, holding a 42.5% share valued at USD 102.8 million. The strong market performance was driven by the region’s higher disease prevalence compared to other continents. This rising incidence encouraged preventive vaccination programs and supported the growing demand for Brucellosis vaccines. The presence of well-developed veterinary systems and efficient disease surveillance further strengthened market performance. The combination of rising awareness and proactive disease control strategies created a favorable environment for vaccine adoption across the region.
The region’s advanced veterinary healthcare infrastructure played a vital role in shaping market growth. Modern research facilities and diagnostic laboratories facilitated innovation in vaccine formulation and efficiency. This technological advancement improved vaccine effectiveness and boosted adoption among livestock owners and veterinary professionals. Regulatory compliance and high manufacturing standards ensured the availability of safe and reliable vaccines. Such developments positioned North America as a hub for vaccine innovation and reinforced its leadership in the global Brucellosis Vaccines Market.
North America’s strict regulatory framework further contributed to its dominant market share. Government agencies enforced rigorous safety and quality standards for vaccine production and distribution. These regulations enhanced stakeholder confidence, promoting large-scale adoption across veterinary sectors. Moreover, continuous investment in research and development led to improved vaccine technologies. Collaboration among pharmaceutical companies, research organizations, and government bodies strengthened production capabilities and optimized the regional supply chain, ensuring consistent vaccine availability across key livestock-producing regions.
Economic stability and increased awareness of zoonotic disease risks also fueled market growth. Livestock owners and veterinarians became more conscious of the economic impact of Brucellosis on animal productivity and trade. Public education campaigns encouraged vaccination as a cost-effective preventive measure. Rising disposable incomes and favorable GDP trends supported higher spending on animal health solutions. Collectively, these factors reinforced North America’s position as a key driver in the global Brucellosis Vaccines Market, with continued growth expected in the coming years.
Segmentation Analysis
In 2023, the Brucellosis Vaccines Market displayed strong segmentation across product types. The RBS1 Vaccine segment led the market, securing over 36.6% share, highlighting its widespread acceptance among healthcare professionals. The S19 Vaccine category also made significant progress, reinforcing the diversity within the product portfolio. These vaccines have enabled the industry to address specific immunization needs effectively. The existence of multiple vaccine options indicates the industry’s commitment to enhancing disease prevention, ensuring better accessibility, and promoting animal health globally.
The market also presented a dynamic vaccine type landscape. The DNA Vaccine segment dominated with over 32.4% share in 2023, supported by growing trust in its advanced immunization mechanism. Subunit Vaccines followed, earning attention for their precision and safety in targeting Brucellosis antigens. Vector and Recombinant Vaccines further contributed by diversifying immunization approaches. This variety underscored ongoing innovation within the market. Collectively, these vaccine types strengthened the industry’s focus on effective, safe, and science-driven preventive solutions.
By application, the Cattle segment held a commanding 56.5% share in 2023, driven by the global emphasis on bovine health. Rising awareness among farmers and government-led vaccination programs supported this growth. The Sheep and Goat segment also showed notable momentum, reflecting expanding vaccination initiatives for small ruminants. Other livestock categories contributed steadily, signifying a broad commitment to comprehensive animal disease management. This collective focus highlights efforts to mitigate economic losses and promote sustainable livestock farming across regions.
In distribution, Veterinary Hospitals and Clinics led the market with an impressive 76.9% share in 2023. Their dominance was supported by accessibility, professional trust, and expertise in vaccination services. Retail channels, including pet stores and pharmacies, accounted for a smaller yet vital portion of vaccine distribution. Emerging digital and unconventional sales platforms also played a modest role in expanding vaccine reach. Going forward, Veterinary Clinics are expected to maintain leadership, supported by professional reliability and growing awareness of animal disease prevention.
Key Market Segments
Product Type
- RBS1 Vaccine
- S19 Vaccine
- Others
Vaccine Type
- DNA Vaccine
- Subunit Vaccine
- Vector Vaccine
- Recombinant Vaccine
Application
- Cattle
- Sheep & Goat
- Others
Distribution Channel
- Veterinary Hospitals & Clinics
- Retail Channels
- Others
Key Players Analysis
The Brucellosis Vaccines Market is characterized by the presence of several established and research-driven players who contribute to innovation and global disease control. Among these, Merck & Co. plays a leading role through its extensive research and strong distribution capabilities. The company’s focus on product innovation and vaccine quality ensures high efficacy and reliability. Its continuous advancements in vaccine development and global outreach enable it to address critical public health challenges effectively, strengthening its influence in the market.
A strong emphasis on research and product improvement defines another major participant, CZ Vaccines. The company’s expertise lies in the development of effective formulations that meet international safety standards. Its commitment to innovation and regulatory compliance ensures product excellence and market credibility. Through ongoing R&D initiatives, CZ Vaccines enhances vaccine performance and contributes to global immunization efforts, reinforcing its role as a trusted and scientifically driven organization in the Brucellosis Vaccines Market.
The market also benefits from the contributions of Colorado Serum Company, recognized for its consistent quality standards and focus on veterinary vaccine manufacturing. The company’s comprehensive product portfolio and production capabilities strengthen vaccine availability across regions. Its adherence to stringent manufacturing protocols ensures product safety and effectiveness. By combining technical expertise with decades of experience, Colorado Serum Company plays a vital role in maintaining the global supply of dependable Brucellosis vaccines.
Emerging and regional manufacturers further diversify the market landscape. Indian Immunologicals, Hester Biosciences, Veterinary Technologies Corporation, Laboratorios Tornel, Fivet Animal Health, and VETAL Animal Health Products Inc. collectively contribute to the market’s expansion. Their efforts focus on improving vaccine accessibility, affordability, and regional distribution. Through innovation, partnerships, and technological upgrades, these companies strengthen market competitiveness and drive the overall advancement of the Brucellosis Vaccines Market worldwide.
Market Key Players
- Merck & Co.
- CZ Vaccines
- Colorado Serum Company
- Indian Immunologicals
- Hester Biosciences
- Veterinary Technologies Corporation
- Laboratorios Tornel
- Fivet Animal Health
- VETAL Animal Health Products Inc.
FAQ
FAQs — Brucellosis Vaccines
1. What is brucellosis and why is vaccination important?
Brucellosis is a bacterial disease that affects animals and humans. It causes abortions, infertility, and reduced productivity in livestock. The disease spreads through contact with infected animals or contaminated products. Vaccination is vital to protect herds and prevent human transmission. It helps build immunity in animals, reducing infection rates and economic losses. Regular vaccination programs, supported by veterinary authorities, play a major role in controlling outbreaks and maintaining healthy livestock populations.
2. What are the main types of brucellosis vaccines used globally?
Two common vaccines are used worldwide — the S19 and RB51 strains. Both are live attenuated vaccines developed to protect cattle from Brucella abortus. For sheep and goats, the Rev.1 strain is widely used. Newer vaccine types, such as DNA, recombinant, and subunit vaccines, are being studied. These advanced options aim to improve safety, minimize diagnostic interference, and provide broader protection. The choice of vaccine depends on animal species, disease prevalence, and regional vaccination policies.
3. How do these vaccines function?
Brucellosis vaccines work by stimulating an animal’s immune system to recognize Brucella bacteria. Once vaccinated, the body develops immune memory that helps fight future infections. The vaccine triggers both antibody and cellular immune responses, reducing disease severity and bacterial shedding. As a result, vaccinated herds show lower infection rates and slower disease transmission. This preventive approach strengthens livestock health, improves productivity, and helps protect people working closely with animals from zoonotic infection risks.
4. Are these vaccines completely effective?
Brucellosis vaccines are highly useful but not 100% effective. Their protection level usually ranges from 70% to 85%, depending on herd health and exposure conditions. Vaccinated animals are less likely to get infected and, if infected, often show milder symptoms. Success also depends on correct dosing, proper administration, and vaccination timing. When vaccination is combined with hygiene control and testing programs, the disease burden drops significantly. Continuous vaccination helps maintain herd immunity and protect regional livestock industries.
5. What are the major limitations of existing vaccines?
Current brucellosis vaccines have several limitations. Some may cause mild infections or abortions in pregnant animals because they are live attenuated. They can also interfere with diagnostic tests, making it hard to identify infected animals. Handling the vaccine requires safety precautions to prevent human exposure. Cold chain management is necessary for maintaining potency. Despite these drawbacks, vaccines remain the most reliable control tool. Ongoing research focuses on improving safety, efficiency, and diagnostic compatibility.
6. At what age should animals be vaccinated?
Heifer calves are usually vaccinated between four and twelve months of age. At this stage, they respond best to vaccination and develop long-lasting immunity. Adult vaccination is sometimes allowed in high-risk zones under veterinary supervision. The exact schedule may vary by country and livestock type. Farmers should follow local animal health guidelines to ensure proper timing and documentation. Early vaccination helps reduce disease transmission and supports national brucellosis eradication efforts.
7. Can pregnant animals be vaccinated?
Vaccination of pregnant animals is generally discouraged. Live vaccines such as S19 or RB51 can occasionally cause abortion or infect the fetus. To avoid this, vaccination is usually carried out before breeding age. In special cases, veterinarians may use alternative vaccines or carefully monitor animals during administration. Proper scheduling ensures protection without harming reproduction. Following veterinary advice is essential for safe vaccination and maintaining herd productivity while minimizing any potential risk.
8. What are the safety precautions during vaccination?
Strict safety precautions are essential during brucellosis vaccination. Handlers must wear gloves, protective eyewear, and masks to prevent accidental exposure. Vaccines like RB51 can infect humans and require immediate medical attention if exposure occurs. Syringes should be handled carefully and disposed of properly. Vaccination must only be performed by trained personnel in accordance with veterinary protocols. Safe handling protects workers, ensures vaccine quality, and prevents accidental infection in humans or unintended contamination in livestock facilities.
9. Can vaccination alone eradicate brucellosis?
Vaccination alone cannot fully eradicate brucellosis but significantly reduces its spread. Effective control requires a combined approach involving testing, isolation, culling of infected animals, and strict biosecurity measures. Vaccination reduces the number of new cases and limits transmission between animals. When combined with surveillance and farmer education, it forms a strong disease control strategy. Countries that integrated vaccination with monitoring programs have successfully reduced infection rates and protected public health.
10. Are there new vaccine developments underway?
Yes, researchers are developing next-generation brucellosis vaccines with better safety and higher efficacy. These include DNA, recombinant, and vector-based vaccines. Such innovations aim to eliminate diagnostic interference and provide immunity across multiple Brucella species. Scientists are also exploring vaccines with DIVA capability, which allows differentiation between vaccinated and infected animals. Improved formulations could reduce handling risks and enhance stability. Continued investment in research is expected to produce safer and more effective solutions in the near future.
FAQs — Brucellosis Vaccines Market
1. What factors drive demand for brucellosis vaccines?
Demand for brucellosis vaccines is driven by growing livestock populations, increased disease awareness, and government control programs. Farmers are adopting preventive vaccination to avoid economic losses and ensure herd health. Rising consumption of dairy and meat products has also increased the need for disease-free production. National animal health campaigns and improved veterinary infrastructure are expanding vaccine coverage. Together, these factors are fueling steady market growth and encouraging ongoing innovation in vaccine development and delivery.
2. Which animal segments account for major vaccine usage?
Cattle account for the largest share of vaccine use because brucellosis mainly affects dairy and beef herds. Sheep and goats are also significant segments, especially in regions where small ruminant farming is common. Swine and camels form niche segments with localized demand. Vaccine adoption levels depend on national control programs, livestock density, and disease prevalence. Expanding vaccination coverage across multiple species is essential for controlling cross-transmission and protecting the broader agricultural ecosystem from economic losses.
3. What regional factors influence market growth?
Regional market growth depends on livestock density, disease prevalence, and government initiatives. In Asia and Africa, growing animal populations and public health campaigns are key growth drivers. In Latin America, strong agricultural exports and disease-control policies sustain vaccine demand. Europe and North America focus on maintaining disease-free herds and preventing outbreaks. Each region’s regulatory framework, veterinary infrastructure, and investment capacity shape the overall vaccine adoption rate and determine long-term market performance.
4. What are the main challenges in the brucellosis vaccine market?
The market faces several challenges, including limited farmer awareness, weak cold-chain systems, and uneven vaccine availability. Regulatory barriers delay product approval in some regions. Differentiating vaccinated from infected animals remains a technical issue. Economic constraints in low-income areas also affect vaccination rates. Addressing these challenges requires strong government support, better education programs, and enhanced logistics. Collaboration among manufacturers, veterinarians, and policymakers can overcome these barriers and ensure sustainable vaccine distribution worldwide.
5. What trends are shaping the future of the market?
The future of the brucellosis vaccine market is shaped by technological innovation and growing disease awareness. New vaccine formulations, including DNA and subunit types, promise improved safety and cross-protection. Governments are promoting public–private partnerships to expand vaccination programs. Digital monitoring systems are being introduced to track vaccine coverage. Farmers are increasingly aware of preventive health benefits. These developments indicate a shift toward more integrated, data-driven, and sustainable approaches to livestock immunization worldwide.
6. How does government policy influence market development?
Government policy strongly influences vaccine demand through national disease control and eradication programs. Many countries fund or mandate livestock vaccination to protect agriculture and trade. Subsidies, procurement contracts, and veterinary outreach initiatives support large-scale immunization. Policies focusing on zoonotic disease prevention and food safety also boost adoption. Effective coordination between public agencies and manufacturers ensures vaccine availability, affordability, and compliance. Consistent policy support helps sustain long-term market stability and improve rural animal health outcomes.
7. Who are the key stakeholders in this market ecosystem?
The brucellosis vaccine market involves several stakeholders, including vaccine manufacturers, government agencies, veterinary institutes, and livestock cooperatives. Distributors and field veterinarians ensure that vaccines reach farmers effectively. Research organizations and universities contribute to innovation and safety improvements. Farmers play a key role as end-users responsible for vaccination compliance. Collaboration among all stakeholders ensures reliable vaccine production, efficient delivery, and widespread adoption, creating a strong foundation for sustainable animal disease control programs globally.
8. How is technological innovation impacting vaccine production?
Technological advances are transforming vaccine production through improved strain engineering, formulation, and quality control. Modern biotechnology helps develop safer, more stable, and effective vaccines. Freeze-drying and adjuvant technologies extend shelf life and enhance immune response. Automation and digital tracking improve manufacturing precision and distribution transparency. These innovations reduce costs, increase reliability, and make vaccination more accessible to remote farming areas. Overall, technology is raising efficiency and enabling faster response to disease outbreaks.
9. What ethical and biosecurity considerations apply?
Ethical and biosecurity standards are vital in vaccine development and use. Manufacturers must ensure product safety for animals and humans. Vaccination programs should minimize animal stress and follow humane handling practices. Biosecurity protocols are enforced to prevent accidental release of live strains. Veterinarians and handlers must be trained in safe administration and waste disposal. Transparent communication and regulatory compliance help build public trust. Upholding these standards ensures responsible vaccination and sustainable livestock health management.
10. What is the outlook for the global brucellosis vaccines market?
The global brucellosis vaccines market is expected to maintain steady growth. Rising livestock populations, increased awareness of zoonotic diseases, and national vaccination drives are key contributors. Technological improvements and stronger veterinary infrastructure are enhancing supply efficiency. Continued investment in research and public health initiatives will expand vaccination coverage. Despite existing challenges, the long-term outlook remains positive, driven by the need to protect food security, livestock productivity, and global agricultural trade from disease-related disruptions.
Conclusion
The Brucellosis Vaccines Market is expected to experience steady growth, supported by global initiatives to control zoonotic diseases. Increasing livestock populations, better awareness of food safety, and strong government vaccination programs are strengthening demand. Advances in biotechnology are leading to safer and more efficient vaccines, improving disease management across regions. Collaboration among veterinary authorities, manufacturers, and research institutions is ensuring effective vaccine supply and quality. Despite challenges such as high costs and limited access in remote areas, continued policy support and innovation are expected to drive market expansion. Overall, the market is positioned for consistent progress in enhancing animal health and preventing human infection worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



