Table of Contents
Introduction
In 2023, the Global Biosimulation Market was valued at USD 3.3 Billion and expected to grow USD 10.1 Billion in 2032. Between 2024 and 2032, this market is estimated to register a CAGR of 13.7%.
The biosimulation market is expanding rapidly as the demand for advanced modeling and simulation technologies increases across the healthcare sector. Biosimulation uses computer-based simulations to predict the behavior of biological systems, aiding in drug discovery and development. This technology is crucial in creating models that simulate the effects of drugs in the human body, significantly reducing the need for animal testing. According to reports, the National Institutes of Health (NIH) emphasize the importance of biosimulation in understanding complex biological processes and improving the success rate of clinical trials. This growth is further propelled by the increasing adoption of artificial intelligence (AI) and machine learning (ML) in creating more accurate and predictive biosimulation models.
Several factors contribute to the growth of the biosimulation market. The increasing incidence of chronic diseases and the rising need for personalized medicine drive the demand for more precise and effective treatment options. Biosimulation helps researchers understand disease mechanisms and develop targeted therapies. Furthermore, technological advancements and increased funding from government and private organizations enhance the development of innovative biosimulation tools. The Food and Drug Administration (FDA) supports biosimulation as a critical component in drug development, encouraging its integration into regulatory submissions to expedite drug approval processes.
However, the biosimulation market also faces significant challenges. High costs associated with biosimulation software and services can limit accessibility, particularly for small and medium-sized enterprises. Additionally, the complexity of biological systems requires sophisticated models and skilled professionals to interpret results accurately. Ensuring data accuracy and reliability is essential, as any discrepancies can lead to flawed conclusions. Overcoming these challenges requires continuous investment in research and development and collaboration between industry stakeholders and regulatory bodies to establish standardized protocols and best practices.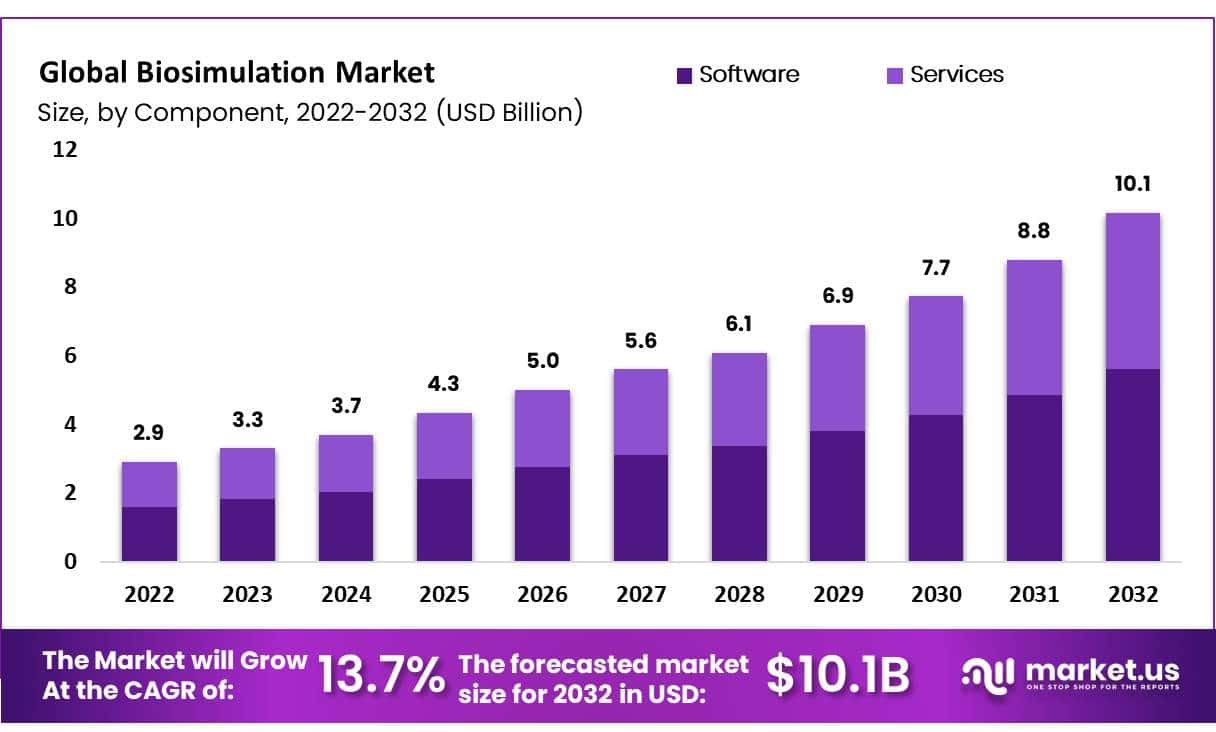
Recent developments in the biosimulation market include the adoption of cloud-based platforms and the integration of real-world data into simulation models. These advancements improve the scalability and flexibility of biosimulation tools, enabling researchers to conduct virtual clinical trials and assess drug interactions more efficiently. As the market evolves, biosimulation is poised to play an increasingly vital role in transforming drug discovery and personalized medicine approaches.
Key Takeaways
- Market Size: In 2023, the Global Biosimulation Market was valued at USD 3.3 Billion and expected to grow USD 10.1 Billion in 2032
- Market Growth: Between 2024 and 2032, this market is estimated to register a CAGR of 13.7%.
- Growth Opportunities: The Expanding Applications and Increased Use of Personalized Medicine
- Delivery Mode Analysis: The Ownership Models Segment Is Expected to Dominate the Market During the Forecast Period
- End-User Analysis: Pharmaceutical and Biotechnology Companies Accounted for A Revenue Share
- Rapid Growth: The biosimulation market is experiencing significant growth due to increasing demand for advanced modeling technologies in drug development and personalized medicine.
- Technological Advancements: Integration of artificial intelligence (AI) and machine learning (ML) is enhancing the accuracy and predictive power of biosimulation models, improving drug discovery processes.
- Regulatory Support: Agencies like the FDA endorse biosimulation as a tool for regulatory submissions, aiding in faster drug approvals and ensuring safety and efficacy.
- Challenges: High costs and the complexity of creating accurate models pose challenges, requiring skilled professionals and substantial investment in research and development.
- Personalized Medicine: Biosimulation plays a critical role in developing targeted therapies and understanding disease mechanisms, supporting the trend towards personalized medicine.
Biosimulation Market Statistics
- Drug Development Time Reduction: Biosimulation can reduce drug development time by 2 to 3 years, leading to faster market entry.
- FDA Drug Approvals: About 90% of new drugs approved by the FDA have used biosimulation in their development process.
- Cost Savings: Implementing biosimulation in drug development can save approximately $100 million per failed drug by identifying inefficacies early.
- Animal Testing Reduction: Biosimulation reduces the need for animal testing by 50%, significantly lowering ethical concerns and costs.
- Regulatory Engagement: Over 20 global regulatory agencies, including the U.S. FDA and European Medicines Agency, now incorporate biosimulation in their review processes.
- Clinical Trial Efficiency: Biosimulation can increase the success rate of clinical trials by 20%, optimizing trial design and reducing patient exposure to ineffective treatments.
- Model Adoption: Over 50% of pharmaceutical companies in the U.S. and Europe use biosimulation models during preclinical and clinical phases.
- Pharmacokinetic Modeling: More than 70% of pharmacokinetic modeling now uses biosimulation to predict drug absorption, distribution, metabolism, and excretion.
- Healthcare Simulation: NIH-supported biosimulation projects have grown by 30% annually, indicating increasing reliance on computational models in healthcare.
- Training Programs: Around 26 universities and multiple healthcare institutions in Europe and the U.S. offer specialized courses in biosimulation.
- Public Sector Investment: The European Union has invested €10.7 million in biosimulation research to advance drug safety and efficacy evaluations.
- FDA Collaboration: The U.S. FDA has partnered with several biosimulation companies to enhance the predictability of drug interactions and adverse effects.
- Software Utilization: Biosimulation software tools are utilized in 60% of the drug development processes in major pharmaceutical companies.
Emerging Trends
- Integration of AI and Machine Learning: Biosimulation models are increasingly integrating artificial intelligence (AI) and machine learning (ML) to enhance predictive accuracy, thereby improving drug discovery and development processes.
- Personalized Medicine: Biosimulation is playing a critical role in the development of personalized medicine by enabling tailored treatment strategies based on individual patient profiles and genetic information.
- Increased Regulatory Acceptance: Regulatory bodies such as the FDA are recognizing biosimulation as a valuable tool for drug development, which is speeding up the approval process for new drugs by providing comprehensive data on safety and efficacy.
- Virtual Clinical Trials: The rise of virtual clinical trials using biosimulation is reducing the need for physical trials, lowering costs, and speeding up the time it takes to bring new therapies to market.
- Enhanced Drug Repurposing: Biosimulation is increasingly used for drug repurposing, identifying new therapeutic uses for existing drugs by simulating their effects on various biological pathways.
- Cost Reduction in Drug Development: By using biosimulation, pharmaceutical companies are able to reduce the high costs associated with traditional drug development processes by predicting outcomes and optimizing experimental designs.
- Improved Predictive Toxicology: The use of biosimulation in predictive toxicology helps in assessing the potential risks of new compounds, ensuring higher safety standards before clinical trials begin.
- Cloud-Based Solutions: The adoption of cloud-based platforms is enhancing the scalability and accessibility of biosimulation tools, allowing for better data integration and collaborative research.
Use Cases
- Drug Development Optimization: Biosimulation models are used to predict how drugs behave in the body, helping to optimize dosage and reduce adverse effects, which accelerates the drug development process.
- Clinical Trial Design: Biosimulation assists in designing clinical trials by predicting outcomes and identifying optimal trial parameters, reducing the need for extensive human testing.
- Personalized Medicine: By modeling individual patient data, biosimulation supports the development of personalized treatment plans, improving patient outcomes by tailoring therapies to individual needs.
- Toxicology Assessment: Biosimulation tools assess the potential toxicity of new compounds, reducing the reliance on animal testing and improving safety evaluations during drug development.
- Regulatory Submissions: Regulatory agencies, such as the FDA, use biosimulation data to support drug approval processes, allowing for faster and more informed decision-making.
- Disease Mechanism Exploration: Biosimulation helps researchers understand complex disease mechanisms, facilitating the development of novel therapeutic approaches for conditions like cancer and neurological disorders.
- Pharmacokinetics and Pharmacodynamics: Models simulate drug absorption, distribution, metabolism, and excretion, providing insights into how drugs interact with biological systems.
- Virtual Clinical Trials: These tools conduct virtual trials that predict clinical outcomes, which reduces costs and accelerates the development timeline for new therapies.
Conclusion
The biosimulation market is rapidly expanding, driven by advancements in AI and ML, and growing demand for personalized medicine. This technology significantly enhances drug discovery and development, reduces reliance on animal testing, and accelerates clinical trials. Despite challenges like high costs and model complexity, regulatory support from agencies like the FDA and NIH underscores its importance. Emerging trends include cloud-based platforms, virtual clinical trials, and improved predictive toxicology. With a projected CAGR of 13.7% from 2024 to 2032, biosimulation is poised to transform healthcare by optimizing drug development and enabling targeted therapies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



