Introduction
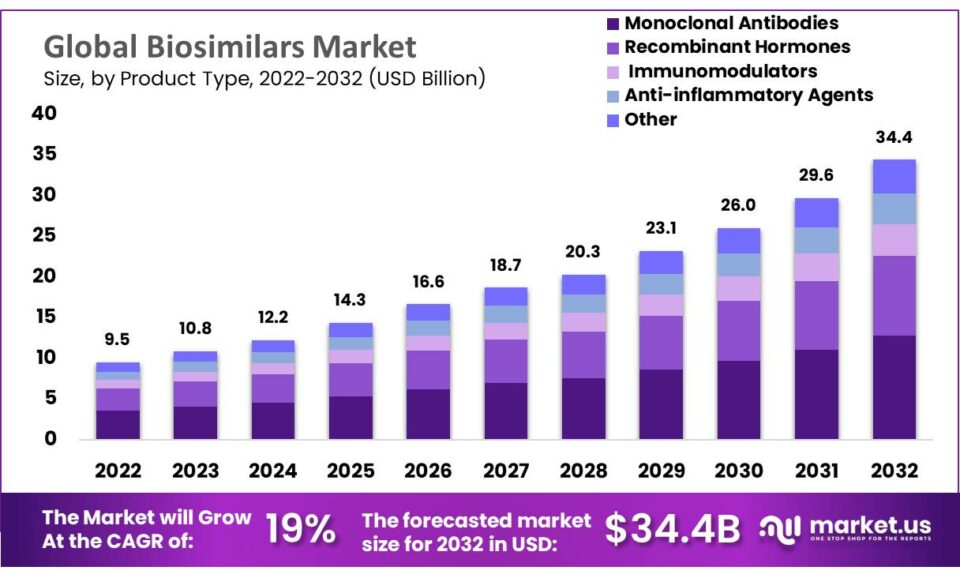
The global biosimilars market, valued at USD 9.5 billion in 2022, is projected to reach approximately USD 34.4 billion by 2032, expanding at a compound annual growth rate (CAGR) of 14.1%. This growth is largely fueled by the need to reduce healthcare costs and enhance access to biological treatments. Biosimilars, closely resembling reference biologic drugs, provide cost-effective alternatives for treating various conditions, including chronic diseases and certain cancers. Their adoption is encouraged by rigorous regulatory standards set by bodies like the FDA and the European Medicines Agency (EMA), ensuring they match their reference products in quality, safety, and efficacy.
In response to growing healthcare demands, regulatory frameworks have evolved to facilitate the quicker adoption of biosimilars. For instance, the FDA has implemented streamlined approval pathways and educational initiatives to boost biosimilar understanding and usage among healthcare providers and patients. The introduction of interchangeable biosimilars, which can be substituted for original biologics without a prescriber’s intervention, mirrors the generic drug model, promoting further integration into healthcare systems.
Recent advancements in the biosimilars sector underscore significant developments by key players. In July 2021, Biocon Ltd announced a partnership to market SEMGLEE, the first interchangeable biosimilar insulin approved by the US FDA. This product is pivotal in diabetes management, offering a high-quality, cost-effective alternative. Additionally, in April 2021, Novartis AG, through its Sandoz division, formed a strategic alliance with Bio-Thera Solutions to globally commercialize a bevacizumab biosimilar, enhancing access to critical oncology treatments.
The market also saw other significant approvals and partnerships aimed at expanding treatment options and managing chronic conditions more effectively. For example, in December 2021, Coherus Biosciences received FDA approval for YUSIMRY, indicated for various inflammatory diseases. Moreover, in May 2021, Viatris, in collaboration with Biocon Biologics, launched Abevmy in Canada, a biosimilar to Avastin used in cancer treatment, pending Health Canada approval.
Overall, the biosimilars market continues to evolve with strategic partnerships and regulatory advancements, providing a broad spectrum of affordable and effective treatment options. This dynamic market not only supports cost management in healthcare but also enhances patient access to necessary treatments, reinforcing its crucial role in the pharmaceutical landscape. The continuous approval of new biosimilars reflects a robust commitment to improving global health outcomes through innovation and regulatory compliance.
Key Takeaways
- Market Forecast: The biosimilars market is expected to grow to USD 34.4 billion by 2032 from USD 9.5 billion in 2022, at a CAGR of 14.1%.
- Product Leadership: Monoclonal antibodies lead the product segment, primarily used for cancer, rheumatoid arthritis, and cardiovascular diseases.
- Top Application: Oncology remains the leading application for biosimilars, fueled by an increasing number of cancer cases.
- Emerging Application: Hormonal deficiencies are becoming a prominent area for biosimilar application.
- Regional Dominance: Europe leads the market, while North America is poised for substantial growth due to increasing biosimilar acceptance and rising cancer incidences.
- Key Players: Notable market participants include Novartis, Amgen, Pfizer, Viatris, and Eli Lilly, employing strategies like partnerships and mergers to enhance market presence.
Biosimilars Statistics
- Equivalence Range and Confidence Intervals: Regulatory agencies generally recommend an equivalence range of 80%–125% with 90% confidence intervals for biosimilar drug comparisons.
- Treatment Effect Preservation: To demonstrate biosimilarity, trials are designed to preserve at least 50% of the original treatment effect, maintaining 50% of the historical 2-sided 70% confidence interval’s lower bound.
- Sample Size Requirements:
- For chemotherapy combined with bevacizumab targeting an objective response rate (ORR) of 38%, an initial sample size of 764 is necessary to achieve a power of 85% and a Type I error rate of 5%.
- Considering a 10% dropout rate, the adjusted sample size required is 849 patients.
- If aiming for an ORR of 40%, the necessary sample sizes are 633 for 80% power, 704 for 85% power, and 800 for 90% power.
- Patient Awareness and Concerns:
- In Europe, 36% of inflammatory bowel disease (IBD) patients are aware of biosimilars.
- In Belgium, 49% of patients with rheumatoid arthritis (RA) are familiar with biosimilars, compared to 66% of RA patients in the UK.
- Among European patients on biosimilars, 80% understand what biosimilars are.
- Concerns among IBD patients familiar with biosimilars include safety (47%) and efficacy (39%); 25% express no specific concerns.
- Treatment Preferences and Nocebo Effect:
- 28% of European IBD patients knowledgeable about biosimilars have requested to switch back to the reference infliximab, despite 44% reporting no increase in disease activity.
- Among 125 patients with IBD or rheumatic disease, 12.8% experienced a nocebo effect after transitioning from reference product to biosimilar infliximab.
Emerging Trends
- Cost-Effectiveness: Biosimilars are increasingly recognized as an economical choice in healthcare, particularly as a substitute for more expensive biologics. This shift is fueled by the expiration of patents on key biologic medications, paving the way for more manufacturers to enter the market. Consequently, this competition drives down prices, making treatments more accessible financially. This trend is particularly significant in markets where cost constraints limit access to essential therapies.
- Regulatory Advancements: Recent improvements in the regulatory landscape, specifically in analytical methods, are pivotal for the development of biosimilars. These advancements enable developers to demonstrate that their products are comparable to well-established biologics without duplicating the original manufacturing processes. Such regulatory progress facilitates more streamlined development and approval processes, enhancing market entry opportunities for new biosimilars.
- Expanded Access: Biosimilars contribute significantly to broadening patient access to vital treatments. By offering lower-cost alternatives to biologics, biosimilars make it feasible for more patients to receive necessary therapies, especially in managing chronic and severe conditions. This expanded access can lead to better health outcomes across a wider demographic, reflecting the critical role of biosimilars in contemporary healthcare strategies.
- Innovative Manufacturing: The biosimilars industry is witnessing a shift towards innovative manufacturing techniques, including the adoption of single-use systems. These technologies not only cut down production costs but also add flexibility in manufacturing operations, making it easier to scale production up or down based on demand. Such advancements are essential for the economic and efficient production of biosimilars, ensuring they remain a competitive and viable option in the pharmaceutical market.
Use Cases
- Chronic Disease Management with Biosimilars: Biosimilars play a pivotal role in managing chronic diseases, including cancer, diabetes, and various autoimmune disorders. As cost-effective alternatives to biologics, biosimilars enhance patient accessibility to essential treatments. This affordability factor is crucial, particularly in long-term disease management, where patients require sustained care. By providing similar therapeutic benefits at a lower cost, biosimilars ensure that more patients can afford the treatments they need without compromising on quality.
- Impact of Biosimilars in Oncology: In oncology, biosimilars have emerged as a game changer by offering substantial cost reductions while maintaining the efficacy of existing cancer treatments. For instance, numerous biosimilars equivalent to the cancer drug Herceptin are now available. These biosimilars are used to treat HER2-overexpressing breast cancer, providing patients with more treatment options and reducing the financial burden of cancer care. The introduction of biosimilars in this field not only expands access but also encourages competition, potentially driving down prices further.
- Biosimilars in Treating Chronic Eye Diseases: Biosimilars have significant implications for treating chronic eye diseases such as age-related macular degeneration and diabetic retinopathy. These conditions, if left untreated, can lead to severe vision loss. Biosimilars offer a more accessible treatment option due to their lower cost, making crucial medications available to a broader range of patients. This increased accessibility is vital for preventing the progression of eye diseases and maintaining patients’ quality of life.
- Biosimilars for Autoimmune Disorders: For individuals suffering from autoimmune disorders like psoriasis and rheumatoid arthritis, biosimilars provide an effective and affordable treatment alternative. These biosimilars deliver comparable outcomes to their reference biologics, but at a reduced cost, making advanced treatments more accessible to patients previously hindered by high prices. The availability of biosimilars not only broadens access but also offers hope to those who might have foregone treatment due to cost constraints.
Conclusion
In conclusion, the biosimilars market is set for substantial growth, driven by the global need for affordable healthcare solutions and enhanced treatment accessibility. With rigorous regulatory support ensuring the safety and efficacy of these products, biosimilars are becoming integral to healthcare systems worldwide. The market’s expansion is further propelled by strategic collaborations and innovations in manufacturing processes. These developments not only enhance patient access to critical therapies but also promise significant cost savings across healthcare systems. As the industry continues to evolve, biosimilars are poised to play a crucial role in shaping the future of medical treatment, making essential medications more accessible and affordable for a broader audience.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



