Table of Contents
Overview
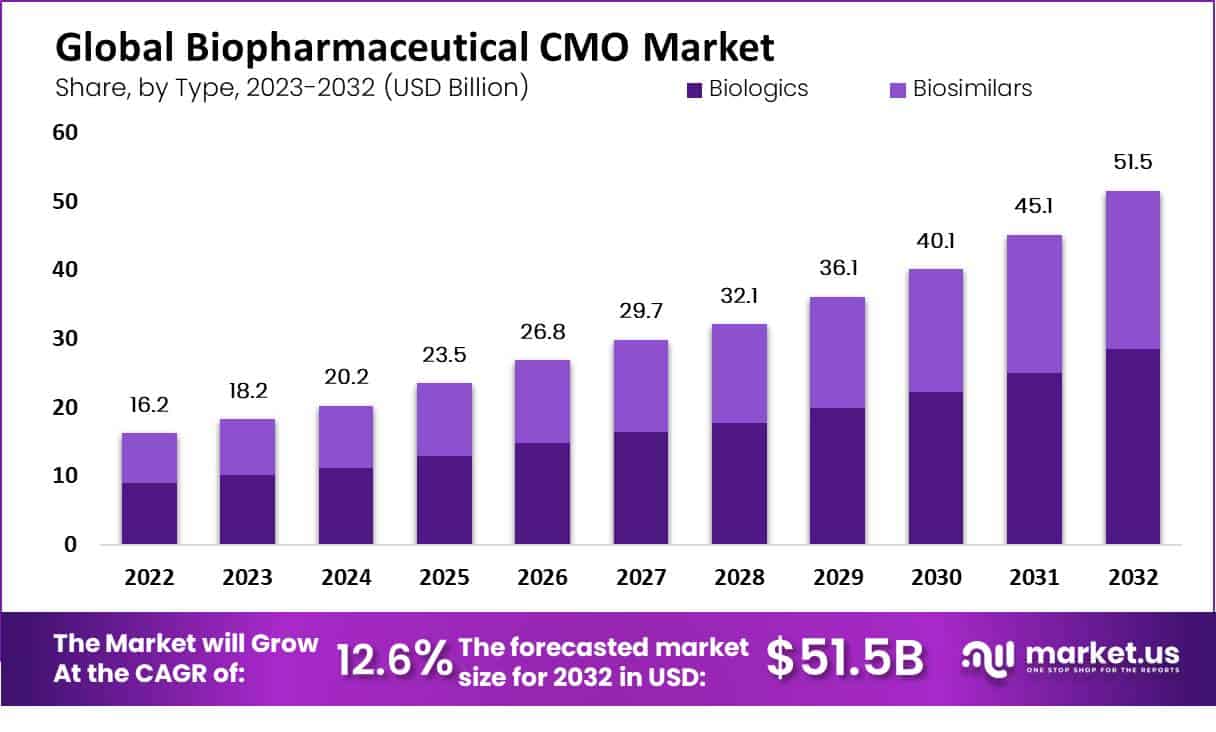
New York, NY – Sep 15, 2025 – The Global Biopharmaceutical CMO Market size is expected to be worth around USD 51.5 Billion by 2032 from USD 20.2 Billion in 2024, growing at a CAGR of 12.6% during the forecast period from 2025 to 2032.
BioGenix Pharma, a leading provider of biopharmaceutical solutions, today announced the expansion of its contract manufacturing organization (CMO) capabilities to meet the increasing global demand for biologics and advanced therapies.
The expansion strengthens the company’s manufacturing facilities, focusing on biologics development, large-scale production, and end-to-end support for pharmaceutical and biotechnology partners. With advanced expertise in mammalian and microbial expression systems, BioGenix Pharma ensures scalable, high-quality, and regulatory-compliant solutions for clients worldwide.
The global biopharmaceutical CMO market has witnessed rapid growth due to rising outsourcing trends, demand for biosimilars, and the advancement of personalized medicines. BioGenix Pharma’s extended portfolio now includes process development, formulation, analytical testing, and commercial-scale production. These services enable partners to accelerate time-to-market while maintaining strict safety and quality standards.
“Our commitment is to deliver innovative, flexible, and sustainable manufacturing solutions that support our clients’ mission of bringing life-saving therapies to patients across the globe,” said Dr. Elena Fischer, Chief Executive Officer of BioGenix Pharma. “With this expansion, we reinforce our position as a trusted partner in the global biopharmaceutical supply chain.”
BioGenix Pharma remains dedicated to global regulatory compliance and sustainable operations, ensuring long-term value creation for clients, healthcare providers, and patients.
Key Takeaways
- Specialization and Expertise: Many contract manufacturing organizations (CMOs) concentrate on specialized areas and technologies, such as cell therapy, gene therapy, viral vector production, advanced bioprocessing, or tailored dosage forms. By cultivating deep expertise in these domains, CMOs provide critical support to clients operating in highly complex therapeutic niches.
- Emergence of Virtual CMOs: A growing trend is the rise of “virtual CMOs,” which deliver project management and oversight through a network of established manufacturing partners. This model enhances agility and flexibility in biopharmaceutical development, enabling companies to optimize resources without investing in full-scale infrastructure.
- Niche Market Focus: Certain CMOs target niche segments, including orphan drugs and therapies for rare diseases. These markets often demand unique production processes at smaller scales, where specialized CMOs offer significant value.
- Diversification of Global Supply Chains: To mitigate risk and ensure uninterrupted supply, biopharmaceutical companies are increasingly diversifying their global supply chains. Partnering with CMOs across multiple regions enables stronger resilience and reduces exposure to regional disruptions.
- Flexible Manufacturing Capacity: CMOs frequently provide adaptable production capacity, allowing clients to scale operations up or down in response to changing demand. This flexibility is a critical asset in managing market fluctuations and supply variability.
- Advantages of Emerging Markets: Emerging markets present unique opportunities for CMOs, including lower production costs, access to expanding patient populations, and valuable local market insights. These factors position emerging regions as attractive hubs for biopharmaceutical manufacturing partnerships.
Regional Analysis
North America accounted for the largest revenue share of 34.2% in 2022. This dominance can be attributed to the strong presence of numerous service providers in the region, as well as the fact that many U.S.-approved products are manufactured by CMOs. The United States continues to lead the global biopharmaceutical industry, supported by a robust base of contract manufacturers. Small and medium-sized pharmaceutical developers, often constrained by limited investments, increasingly outsource production to CMOs, further strengthening the regional market.
Asia is emerging as a key driver of outsourcing growth, primarily due to lower labor and operational costs. Countries such as China, India, Japan, and South Korea are becoming major players, supported by expanding infrastructure and cost-effective manufacturing capabilities. India, in particular, is expected to demonstrate significant progress, driven by large-scale molecule production, which not only fuels domestic growth but also contributes to the broader economy.
Latin America and the Middle East & Africa currently hold smaller shares of the market. However, both regions are poised for notable expansion. Growth will be supported by the establishment of new manufacturing facilities and the entry of multiple small-scale CMOs, which will enhance local capabilities.
The countries covered in the biopharmaceutical CMO market report include Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, the U.K., and the U.S.. Across these markets, factors such as the rapidly growing geriatric population, increasing per capita income, high patient volumes, and greater healthcare awareness are expected to accelerate the adoption of CMO services globally.
Frequently Asked Questions on Biopharmaceutical CMO
- What is a Biopharmaceutical CMO?
A biopharmaceutical contract manufacturing organization (CMO) provides outsourcing services for drug development and production. These organizations assist pharmaceutical and biotech companies in scaling operations, ensuring regulatory compliance, and delivering high-quality products without significant capital investments in infrastructure. - What services do Biopharmaceutical CMOs provide?
CMOs offer services such as process development, formulation, analytical testing, cell line development, and large-scale biologics manufacturing. They also provide flexible production capacity, regulatory support, and packaging solutions to help clients accelerate commercialization of therapies. - Why are Biopharmaceutical CMOs important?
Biopharmaceutical CMOs are vital as they allow pharmaceutical companies to focus on core research while outsourcing complex, capital-intensive manufacturing. Their specialized expertise and advanced facilities ensure efficient production of biologics, biosimilars, and advanced therapies, reducing risks and development timelines. - What are examples of Biopharmaceutical CMO specialization?
Many CMOs specialize in areas such as cell therapy, gene therapy, viral vector production, and complex biologics. These niche expertise areas enable them to meet the unique demands of cutting-edge treatments and support clients targeting highly specific therapeutic segments. - What are Virtual CMOs?
Virtual CMOs operate by leveraging networks of established manufacturing partners rather than owning production facilities. They provide project management, oversight, and coordination, offering clients agility and cost efficiency in biopharmaceutical development while reducing dependency on physical infrastructure. - How large is the Biopharmaceutical CMO market?
The global biopharmaceutical CMO market is expanding steadily, driven by rising demand for biologics, biosimilars, and advanced therapies. North America currently holds the largest share, while Asia is emerging rapidly due to lower costs and growing infrastructure. - Which regions dominate the Biopharmaceutical CMO market?
North America dominates with strong infrastructure and numerous service providers. Asia is witnessing the fastest growth due to cost efficiencies and manufacturing capacity, while Europe remains a critical hub for innovation, compliance, and advanced therapeutic development partnerships. - What role do emerging markets play in the Biopharmaceutical CMO industry?
Emerging markets such as India, Brazil, and South Korea offer advantages like lower production costs, expanding patient populations, and new investment opportunities. These regions are becoming attractive outsourcing destinations, strengthening global supply chain diversification strategies.
Conclusion
The biopharmaceutical CMO industry is experiencing robust growth, driven by rising demand for biologics, biosimilars, and advanced therapies. North America continues to dominate, while Asia is rapidly emerging as a competitive hub due to cost advantages and expanding infrastructure. Latin America, the Middle East, and Africa are also showing promising potential with increasing investments and new facilities.
CMOs’ specialization in niche therapies, adoption of flexible capacity models, and the emergence of virtual CMOs are further transforming the landscape. With expanding global supply chains and strong participation from emerging markets, CMOs are set to remain integral to biopharmaceutical advancement worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



