Table of Contents
Overview
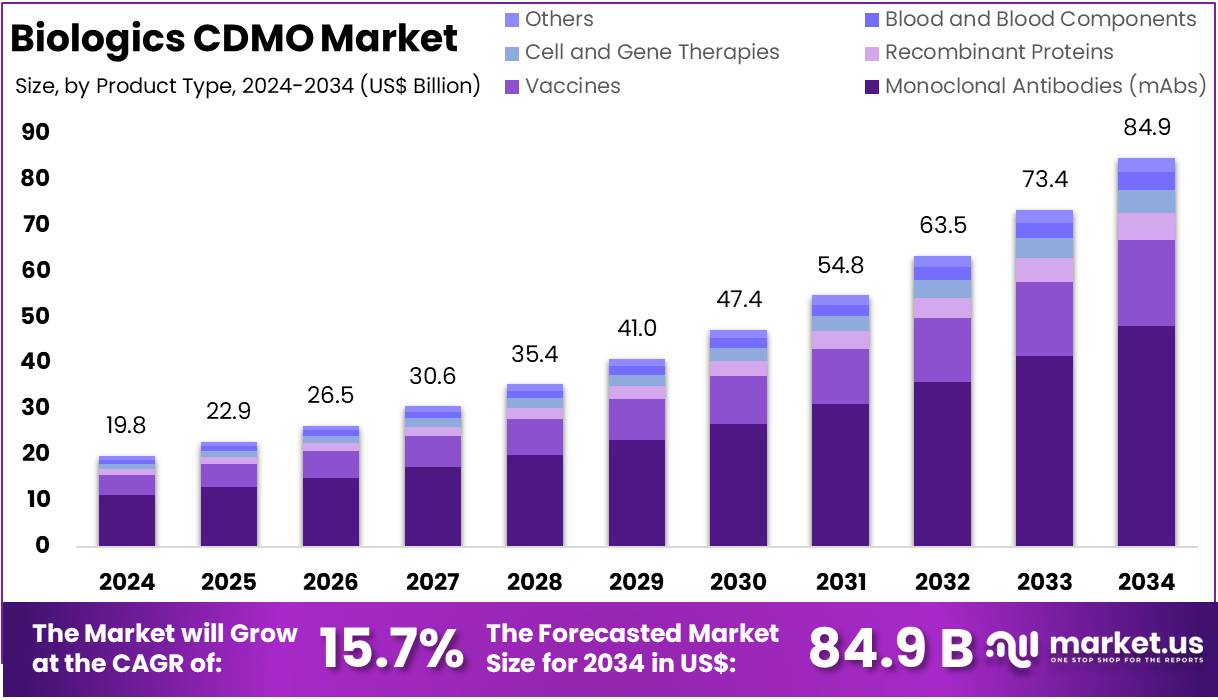
New York, NY – Aug 04, 2025 : The Global Biologics CDMO Market is projected to reach US$ 84.9 Billion by 2034, growing from US$ 19.8 Billion in 2024. This marks a CAGR of 15.7% from 2025 to 2034. North America leads the market, accounting for more than 40.9% share, with a value of US$ 8.08 billion in 2024. The growth is largely driven by the rising demand for biologic therapies. These include monoclonal antibodies, vaccines, and other complex treatments for cancer, autoimmune diseases, and chronic conditions like diabetes.
Biologic drugs are known for their high efficacy and safety, as highlighted by the WHO. However, their high cost limits access in many regions. This has led to a surge in biosimilars, which offer affordable alternatives to branded biologics. Biosimilars undergo strict testing to ensure they are as effective and safe as the original drugs. As pharmaceutical firms grow their biosimilar pipelines, CDMOs are increasingly being tapped for development and production support. This shift opens significant growth opportunities across the biologics CDMO space.
Technological innovation is also shaping the biologics CDMO landscape. Advances in cell culture, purification, and analytical tools have improved production efficiency. These new platforms offer better batch consistency and reduce manufacturing costs. CDMOs are adopting such innovations to meet rising global demand for biologic treatments. By leveraging these tools, CDMOs are expanding their service capabilities. This makes them attractive partners for both large pharmaceutical firms and emerging biotech companies aiming for scalable and reliable production.
Supportive regulatory frameworks are further accelerating CDMO market growth. The U.S. FDA and WHO have issued guidelines that simplify chemistry, manufacturing, and control (CMC) requirements. These standards improve product quality and streamline development timelines. Faster approvals make it easier for drug makers to bring biologics and biosimilars to market. CDMOs benefit by offering services that align with these evolving regulatory needs. This compliance strength gives CDMOs a competitive edge, especially as more firms outsource biologics development and manufacturing.
The COVID-19 pandemic spotlighted the critical role of CDMOs. Vaccine output jumped from 1.5 billion to over 11 billion doses by 2021. CDMOs partnered with major pharma companies like Pfizer-BioNTech and Moderna to meet this surge. Their flexibility and manufacturing strength were vital in meeting global demand. A past FDA study showed that 62% of U.S. drug shortages were linked to quality issues. CDMOs help address this by offering quality-focused production. Their role in global biologics supply continues to expand due to their scale, speed, and compliance.
Key Takeaways
- A market expert stated that the global biologics CDMO sector is expected to hit nearly US$ 84.9 billion by 2034, from US$ 19.8 billion.
- Industry professionals note the market is expanding at a CAGR of 15.7% from 2025 to 2034, driven by outsourcing demand in biologics.
- According to analysts, monoclonal antibodies (mAbs) led the product segment in 2024, contributing over 56.7% of the total biologics CDMO market share.
- Experts highlight that therapeutic applications dominated in 2024, comprising more than 80.4% of the market due to their use in treating chronic diseases.
- In 2024, the opioids drug class held a 50.7%+ market share, showing its dominant role in biologics CDMO outsourcing, according to researchers.
- Market observers reported North America as the top-performing region in 2024, generating over US$ 8.08 billion and holding a 40.9% market share.
Regional Analysis
In 2024, North America held a strong lead in the global biologics CDMO market, with a 40.9% share and a market value of US$ 8.08 billion. This dominance is driven by the region’s advanced pharmaceutical and biotechnology sectors. Many top drug manufacturers in the U.S. and Canada rely on CDMOs for biologics development. Outsourcing trends are growing, boosting regional market strength. The U.S. benefits from supportive regulations and faster drug approvals. CDMO firms are expanding their services, particularly for monoclonal antibodies and cell therapies.
Another major factor behind North America’s growth is its strong infrastructure and innovation ecosystem. New production facilities are being built to meet rising demand. The high burden of chronic diseases is increasing the need for personalized biologics. This pushes companies to partner with CDMOs to reduce costs and speed up development. Public-private partnerships and government funding support early-stage research and commercialization. Academic and industry collaborations also drive innovation. These combined forces are expected to keep North America ahead in the biologics CDMO market.
Segmentation Analysis
In 2024, Monoclonal Antibodies (mAbs) dominated the Biologics CDMO market, accounting for over 56.7% of the product type segment. Their high usage in cancer and autoimmune disease treatments drives this dominance. mAbs are valued for their targeted action and minimal side effects. Ongoing advancements in personalized medicine continue to boost their demand. Additionally, rising biologics approvals increase the need for mAb manufacturing services. This segment remains a major focus for CDMOs due to its scalability and therapeutic relevance.
Vaccines held the second-largest share in the product segment. Demand surged with global immunization efforts and pandemic response strategies. CDMOs are essential for vaccine production, especially mRNA and viral vector platforms. Recombinant proteins also showed solid growth, used mainly in managing diabetes and hormone deficiencies. Innovation in protein expression systems is driving this segment forward. These products benefit from strong research pipelines and public health funding. Together, they contribute significantly to the expanding biologics manufacturing landscape.
Therapeutic applications led the Biologics CDMO market by capturing over 80.4% share in 2024. This was driven by high demand for biologics in treating chronic and infectious diseases. Monoclonal antibodies and gene therapies dominate this space due to their targeted treatment potential. Diagnostic and other applications held smaller shares but are growing. Personalized medicine and companion diagnostics are expanding opportunities in diagnostics. CDMOs are now adapting capabilities to meet evolving needs across various end-user groups, including pharmaceutical firms, biotech startups, and public research bodies.
Key Market Segments
By Product Type
- Monoclonal Antibodies (mAbs)
- Vaccines
- Recombinant Proteins
- Cell and Gene Therapies
- Blood and Blood Components
- Others
By Application
- Therapeutic Applications
- Diagnostic Applications
- Others
By End-User
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic and Research Institutions
- Others
Key Players Analysis
The biologics CDMO market is led by key players like Lonza Group, WuXi AppTec, and Samsung Biologics. Lonza holds a strong position with its global biomanufacturing sites and advanced capabilities. It supports both clinical and commercial-scale biologics production. WuXi AppTec offers integrated services with fast development and flexible outsourcing. Samsung Biologics is known for its large-scale capacity and ongoing plant expansions. The company focuses on biosimilars and novel biologics. It uses advanced automation and ensures compliance with regulatory standards to meet client needs.
Boehringer Ingelheim is another major player offering end-to-end biologics CDMO services. It specializes in cell culture and large-scale protein manufacturing using proprietary platforms. Catalent continues to grow its biologics pipeline through acquisitions and new facilities. It provides full-service solutions from development to fill-finish. Both companies emphasize monoclonal antibodies, injectables, and gene therapies. Other key contributors include Fujifilm Diosynth, Thermo Fisher, and AGC Biologics. They focus on recombinant proteins, vaccines, and gene therapies while expanding global capacity and upgrading regulatory compliance systems.
Leading Market Key Players
- Lonza Group
- WuXi AppTec
- Samsung Biologics
- Boehringer Ingelheim
- Catalent
- Recipharm
- Fujifilm Diosynth Biotechnologies
- Vetter Pharma
- Rentschler Biopharma
- CordenPharma
- Minakem
- KBI Biopharma
Emerging Trends
1. Rising Demand for Monoclonal Antibodies (mAbs)
Pharma companies are focusing more on targeted therapies like monoclonal antibodies (mAbs). These biologics are effective in treating cancer, autoimmune disorders, and infections. Because of their precision and fewer side effects, demand is rising fast. CDMOs are responding by investing in advanced facilities to handle the complex production needs of mAbs. These biologics require high technical expertise, making them ideal for outsourcing. CDMOs that offer large-scale and flexible capabilities are seeing more contracts from pharma companies. As biologics become more advanced, the need for specialized CDMO partners is expected to grow even more in the coming years.
2. Shift Toward Personalized Medicine
Biologics are no longer one-size-fits-all. Pharma companies are now developing treatments tailored to specific patients or genetic profiles. This trend is driving the demand for personalized medicines such as cell and gene therapies. CDMOs are adapting by offering flexible, small-batch manufacturing solutions. These setups support fast production without sacrificing quality. Speed and flexibility are critical in personalized treatments. CDMOs are also enhancing their ability to manage complex logistics and regulatory needs. As healthcare moves toward precision medicine, CDMOs with adaptable services will play a key role in delivering patient-specific therapies.
3. Growth in Outsourcing by Biotech Startups
Biotech startups often lack the infrastructure needed for biologics manufacturing. To save time and lower upfront costs, they are turning to CDMOs for help. These partnerships allow startups to focus on research and innovation while CDMOs handle production. The trend is leading to a sharp rise in early-stage development projects being outsourced. CDMOs are now creating flexible service packages to meet the specific needs of smaller firms. From process development to clinical trial manufacturing, CDMOs are becoming essential partners for biotech companies. This shift is helping bring new biologic therapies to market faster.
4. Adoption of Single-Use Technologies
CDMOs are rapidly adopting single-use technologies such as disposable bioreactors and filters. These tools help reduce contamination risks and speed up turnaround times. They also eliminate the need for extensive cleaning, saving time and resources. Single-use systems are ideal for producing small batches of biologics, which are common in early-stage trials and personalized medicine. These technologies also support flexible manufacturing setups, allowing CDMOs to quickly switch between different projects. As demand grows for agile production methods, the use of single-use equipment will become more widespread across biologics facilities.
5. Expansion into Emerging Markets
CDMOs are expanding into fast-growing regions like Asia-Pacific, Latin America, and the Middle East. These areas are seeing a rise in demand for biologics due to growing healthcare needs and better regulatory environments. By setting up local manufacturing facilities or partnering with regional firms, CDMOs can serve clients more efficiently. This also helps reduce shipping costs and meet local compliance requirements. Local presence improves speed to market and builds trust with clients in these regions. As global demand for biologics rises, having a footprint in emerging markets is becoming a key growth strategy.
6. Use of Artificial Intelligence and Automation
AI and automation are transforming how CDMOs operate. Smart systems now monitor production lines in real time, helping to catch errors early and improve product yield. Automation also reduces human error and enhances consistency. These technologies speed up manufacturing and make it easier to scale up production. AI tools are also used to analyze data, predict maintenance needs, and optimize workflows. By embracing digital tools, CDMOs can offer faster turnaround times and better quality control. This tech-driven approach supports both innovation and regulatory compliance in biologics manufacturing.
Use Cases
1. Clinical Trial Support for New Biologics
Pharmaceutical companies often need small batches of biologic drugs for clinical trials. CDMOs (Contract Development and Manufacturing Organizations) help by designing the production process and manufacturing trial-ready batches. They also ensure every step meets strict regulatory guidelines. This support speeds up the clinical development process. It allows pharmaceutical firms to focus on research while the CDMO handles the technical work. By partnering with a CDMO, companies can also avoid the cost of building trial-specific manufacturing facilities. CDMOs provide a flexible, cost-effective way to move from lab to clinic smoothly.
2. Scaling Up Production After Drug Approval
After a biologic drug gets approved, companies must quickly increase production. CDMOs assist by scaling up operations from clinical to commercial levels. This includes technology transfer, optimizing production lines, and ensuring consistent product quality. CDMOs help companies meet growing demand without delays. They also provide large-scale facilities and technical teams experienced in high-volume biologics. This reduces time to market and avoids costly errors. Working with a CDMO ensures smooth scaling while maintaining all required regulatory and quality standards. It’s a reliable way to enter the commercial market.
3. Support for Biosimilar Development
As patents on popular biologics expire, biosimilars are gaining attention. These are similar versions of approved biologic drugs. CDMOs help companies develop biosimilars by reverse-engineering the original product. They then optimize the manufacturing process to ensure safety, purity, and effectiveness. CDMOs have the tools and expertise to manage complex analytics, protein expression, and formulation. This support reduces development time and cost. Partnering with a CDMO also ensures regulatory readiness for global markets. It’s an efficient path for companies wanting to enter the biosimilar space quickly and confidently.
4. Manufacturing for Cell & Gene Therapies
Cell and gene therapies, such as CAR-T and gene editing treatments, are highly advanced. Producing them requires strict conditions and specialized knowledge. CDMOs with expertise in this field offer end-to-end support. They manage living cells, viral vectors, and sterile environments. These therapies often need custom production solutions. CDMOs provide the right technology, trained staff, and quality control systems. They help innovators bring these breakthrough treatments to market faster. By handling the technical side, CDMOs let developers focus on science and patient outcomes. This support is crucial for emerging therapies.
5. Handling Regulatory Documentation and Compliance
Biologics manufacturing follows strict global rules. CDMOs help companies meet these by maintaining GMP (Good Manufacturing Practice) standards. They also handle detailed documentation for regulatory submissions. This includes batch records, safety data, and validation reports. CDMOs have dedicated regulatory teams that stay updated on the latest guidelines. Their support helps prevent costly delays or rejections. Companies can trust CDMOs to keep everything compliant from start to finish. This not only speeds up product approvals but also builds trust with regulators. It’s a key service for global market access.
6. Producing Biologics for Niche or Rare Diseases
Drugs for rare diseases often serve very small patient groups. Making these drugs in-house can be too expensive for many firms. CDMOs offer small-scale, high-quality manufacturing tailored to rare biologics. They use flexible systems to produce limited batches cost-effectively. This makes it easier for companies to supply treatments without building new facilities. CDMOs also provide stability and quality assurance, even with low volumes. Their support makes orphan drug development more practical. As rare disease treatments rise, this service is becoming more valuable for biopharma companies.
7. Disaster Recovery and Backup Manufacturing
Unexpected problems like equipment failure or natural disasters can stop drug production. CDMOs act as backup manufacturing partners to keep the supply chain running. They can quickly take over production if a company’s main site goes offline. This helps prevent drug shortages and protects patient access. CDMOs have the tools, trained staff, and flexible capacity to support emergency needs. Some companies even set up long-term agreements for disaster recovery. It’s a smart way to add resilience to biologics manufacturing. CDMOs offer peace of mind in uncertain times.
Conclusion
In conclusion, the biologics CDMO market is growing fast due to rising demand for complex therapies like monoclonal antibodies, vaccines, and gene therapies. Companies are outsourcing more as they seek flexible, cost-effective, and high-quality manufacturing. CDMOs are meeting this need with advanced technologies, strong regulatory compliance, and global expansion. Trends like personalized medicine and biosimilars are also boosting the market. North America leads, but emerging regions are gaining ground. With increasing partnerships between pharma firms and CDMOs, the market will continue to grow. CDMOs are now essential for bringing biologics to market quickly, safely, and at scale, supporting both innovation and patient care worldwide.
View More Similar Reports
Biologics Market, Cancer Biological Therapy Market, Orthobiologics Market, Biological Safety Testing Market, Spine Biologics Market, Biological Safety Testing Products and Services Market, Biological PCR Technology Market, CDMO Market, Topical Drugs CDMO Market, Small Molecule CDMO Market, Cell and Gene Therapy CDMO Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



