Table of Contents
Overview
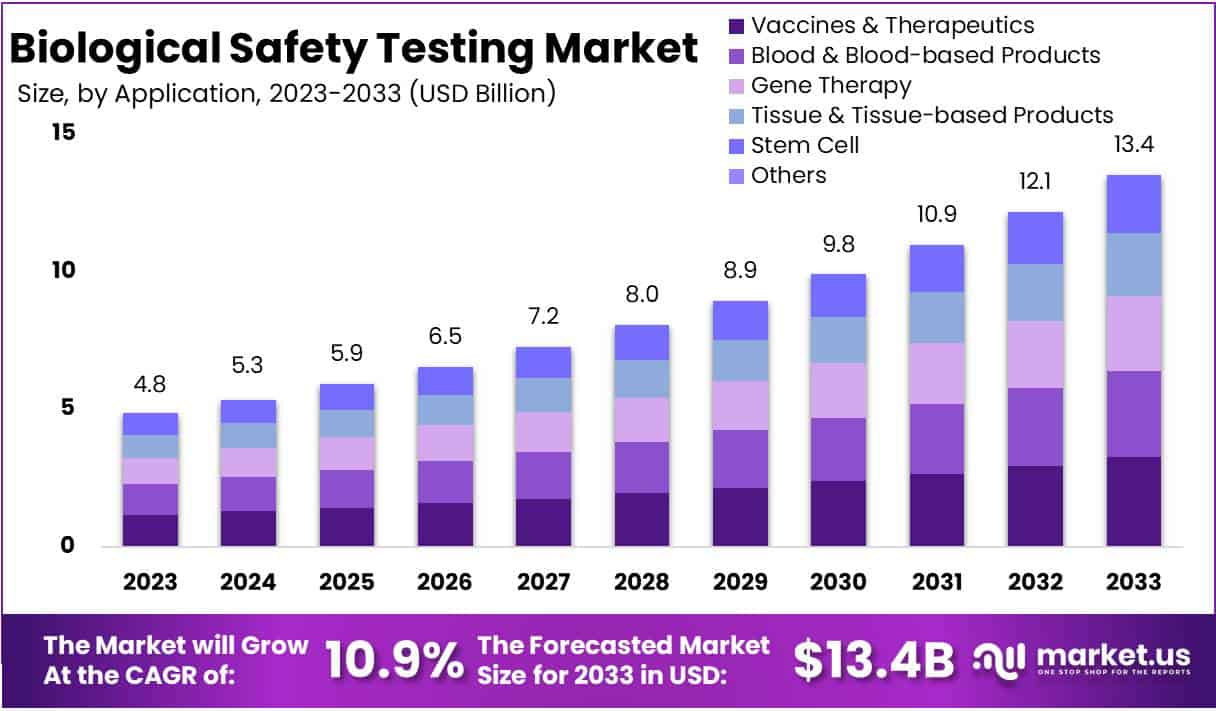
The Biological Safety Testing Market is projected to grow from USD 4.8 billion in 2023 to approximately USD 13.4 billion by 2033, with a robust Compound Annual Growth Rate (CAGR) of 10.9% during the forecast period. Several key drivers are contributing to this growth, including stricter regulatory standards, technological advancements, increased demand from the biopharmaceutical sector, and expanding healthcare markets worldwide.
One of the primary growth drivers is the increasing regulatory requirements across industries such as pharmaceuticals, biotechnology, and medical devices. Regulatory bodies like the FDA, EMA, and WHO enforce stringent safety testing standards to ensure the safety and efficacy of biologics and medical devices. These regulations, including updates to ISO 10993 for medical devices and ICH Q3D guidelines for elemental impurities, create continuous demand for specialized biological safety testing services.
Technological advancements are also fueling the market’s expansion. New testing methodologies, such as in vitro assays, molecular biology techniques, and advanced cell culture models, have improved the accuracy and cost-effectiveness of biological safety tests. Automation and the use of Artificial Intelligence (AI) in data analysis further enhance testing efficiency. These innovations streamline the testing process, reducing both time and costs while improving the reliability of results.
The growing demand for biopharmaceuticals, particularly biologics like monoclonal antibodies and gene therapies, is another key driver. As the biopharmaceutical sector continues to expand, the need for rigorous biological safety testing increases. This trend was amplified by the COVID-19 pandemic, which accelerated vaccine development and heightened the demand for reliable safety testing. Investments in personalized medicine and gene therapies are expected to continue driving the need for comprehensive biological testing services.
Additionally, the global expansion of healthcare infrastructure, especially in emerging markets, presents significant opportunities for market growth. Countries in Asia-Pacific and Latin America are increasing investments in biotechnology and healthcare, driving the demand for biological safety testing services. As these regions adopt international safety standards, they will play a critical role in the global market’s expansion.
In conclusion, the biological safety testing market is poised for substantial growth due to regulatory pressures, technological innovations, and rising demand from the biopharmaceutical and medical device industries. As healthcare services and biopharmaceutical investments increase globally, especially in emerging markets, the market for biological safety testing will continue to expand, providing opportunities for companies offering compliant, innovative testing solutions.
Key Takeaways
- Market Growth Projection: The Biological Safety Testing Market is expected to reach USD 13.4 Billion by 2033, driven by a 10.9% CAGR from 2024 to 2033.
- Product Segment Leadership: In 2023, the Reagents & Kits segment led with 39.7% of the market share, playing a crucial role in ensuring accurate testing processes.
- Application Segment Leadership: Vaccines & Therapeutics captured 24.1% of the market share in 2023, underlining the importance of safety testing in medical product development.
- Test Type Dominance: Endotoxin Tests led the market in 2023, holding a significant 22.6% share, essential for detecting harmful endotoxins and ensuring product safety.
- Key Growth Driver – R&D Investment: Increased funding for innovative biologics and biosimilars is driving the market’s growth, supporting advancements in safety testing procedures.
- Industry Expansion: Global growth in the pharmaceutical and biotechnology sectors is spurring demand for biological safety testing to maintain product safety and regulatory compliance.
- Major Market Challenge – Cost Constraints: High testing costs remain a significant barrier, particularly for smaller companies or those with limited resources for comprehensive safety assessments.
- Opportunity – Outsourcing Trend: The growing trend of outsourcing biological safety testing services presents significant opportunities for service providers to meet rising market demand.
- Technological Advancements: Advancements in testing methods, such as 3D tissue models, are evolving, enhancing the accuracy and effectiveness of biological safety testing processes.
- Regional Market Leadership: North America dominated the market in 2023, holding over 36.2% of the share, supported by advanced healthcare infrastructure and strict regulatory frameworks.
Regional Analysis
In 2023, North America dominated the Biological Safety Testing Market with a market share of over 36.2% and a market value of USD 1.73 billion. The region’s strong healthcare infrastructure plays a key role in this dominance. Strict regulations from organizations like the FDA and Health Canada ensure the safety of pharmaceuticals and biologics, fostering market confidence. This regulatory framework is vital in maintaining the region’s leadership position in biological safety testing.
The growing demand for biological safety testing in North America is also driven by the region’s thriving biopharmaceutical industry. Companies in this sector engage in cutting-edge research, necessitating thorough safety assessments. Furthermore, North America’s focus on technological innovation and research excellence places it at the forefront of safety testing methodologies. This competitive edge enhances the region’s ability to meet evolving safety standards and market needs.
Challenges like the COVID-19 pandemic have further emphasized the importance of rigorous safety testing in North America. The region’s proactive approach to addressing public health crises has accelerated the demand for comprehensive safety measures. With continued investment in diagnostic solutions, North America is well-positioned to lead the market. The combination of regulatory support, innovation, and a commitment to public health ensures the region’s dominance in the Biological Safety Testing Market for the foreseeable future.
Segmentation Analysis
In 2023, the Biological Safety Testing market was dominated by the Reagents & Kits segment, capturing over 39.7% of the market. This segment is crucial in ensuring accurate testing processes by facilitating the detection of various biological elements. Following closely behind, the Instruments segment played a significant role in enhancing the precision and efficiency of tests, contributing notably to the market. Additionally, the Services segment offered essential support, providing expertise and consultation to laboratories, further solidifying its importance in biological safety testing.
The Vaccines & Therapeutics segment led the application analysis in 2023, holding a substantial 24.1% market share. This dominance was driven by the need for rigorous safety testing in vaccines, monoclonal antibodies, and recombinant proteins. Safety assessments are vital for ensuring the quality of these life-saving medical interventions. Furthermore, the Gene Therapy and Stem Cell segments showcased significant growth, highlighting the increasing importance of stringent safety evaluations in these innovative treatments. These advancements underline the market’s ongoing commitment to safety standards.
Test types such as Endotoxin Tests, Sterility Tests, and Bioburden Tests emerged as key players in the market. In 2023, Endotoxin Tests captured over 22.6% of the market, playing a critical role in detecting harmful endotoxins. Sterility and Bioburden Tests were essential in verifying the absence of microorganisms and quantifying microbial load, respectively. Adventitious Agent Detection and Residual Host Contamination Detection Tests also gained prominence, addressing concerns related to contaminants and ensuring product purity in biopharmaceuticals. These tests continue to be integral in ensuring the safety of biological products.
Key Players Analysis
In the Biological Safety Testing Market, Charles River Laboratories stands out as a key player. The company’s expertise in preclinical and clinical research services allows it to provide comprehensive product safety solutions. Their rigorous testing procedures ensure that products meet stringent regulatory standards, making them a trusted partner for biopharmaceutical and biotechnology companies. Their strong focus on innovation and quality positions them as a leader in this growing market.
BSL Bioservice is another significant contributor, specializing in biosafety testing. The company offers comprehensive solutions tailored to the biopharmaceutical and biotechnology sectors. With a strong emphasis on quality and compliance, BSL Bioservice supports its clients in meeting regulatory requirements. This focus on robust testing protocols makes them a reliable partner for businesses seeking safe and compliant biological testing services, enhancing their market presence and reputation.
Merck KGaA, operating as MilliporeSigma, also plays a crucial role in the Biological Safety Testing Market. The company is known for its cutting-edge technologies and global influence. MilliporeSigma’s advanced biopharmaceutical manufacturing solutions set industry standards, contributing to the market’s growth. Their commitment to innovation drives progress in biological safety testing, making them a leading force in advancing biomanufacturing processes and ensuring safety across the industry.
Market Key Players
- Charles River Laboratories
- BSL Bioservice
- Merck KGaA (MilliporeSigma)
- Samsung Biologics
- Sartorius AG
- Eurofins Scientific
- SGS Société Générale de Surveillance SA
- Thermo Fisher Scientific Inc.
- BIOMÉRIEUX
- Lonza
FAQ
FAQs on Biological Safety Testing
1. What is biological safety testing?
Biological safety testing involves testing biological products like vaccines, medical devices, and biologics to ensure they are safe for use. It checks for harmful contaminants such as bacteria, viruses, or toxins that could pose risks to humans or animals. The testing process ensures that products are free from biological hazards. This process is crucial for protecting public health and ensuring that the products meet regulatory standards set by health authorities.
2. Why is biological safety testing important?
Biological safety testing is important because it ensures that biological products, such as vaccines and medical devices, are free from harmful contaminants. These tests detect viruses, bacteria, or toxins that could harm humans, animals, or the environment. Ensuring safety helps prevent serious health risks and ensures that products comply with global health standards. Without these tests, unsafe products could enter the market, causing public health issues. Therefore, testing is essential for both product safety and consumer protection.
3. What are the common types of biological safety tests?
Common biological safety tests include sterility testing, endotoxin testing, and cytotoxicity testing. Sterility tests check for microbial contamination, while endotoxin testing identifies harmful toxins. Cytotoxicity testing ensures that a product does not cause cell damage. Hemocompatibility testing evaluates how products interact with blood, and mutagenicity testing checks for genetic damage. These tests are essential for assessing the safety of biological products like medical devices, vaccines, and biologics before they reach consumers or patients.
4. What are the regulatory standards for biological safety testing?
Biological safety testing is regulated by guidelines such as Good Laboratory Practice (GLP), ISO 13485, and U.S. Pharmacopeia standards. Regulatory agencies like the FDA, EMA, and WHO set these standards to ensure product safety. These regulations ensure that testing is consistent, accurate, and reliable. Compliance with these standards is crucial for obtaining approval to market biological products. Regulatory frameworks also ensure that the testing process adheres to the highest safety and quality control measures required in the industry.
5. Who performs biological safety testing?
Biological safety testing is usually performed by specialized laboratories or contract research organizations (CROs). These laboratories are equipped with the necessary expertise and facilities to conduct testing. They are typically accredited by regulatory bodies like ISO or GLP to ensure that they meet high standards. CROs often collaborate with pharmaceutical and medical device companies to conduct the required tests. These professionals play a key role in ensuring that products are safe before being released into the market.
6. How is biological safety testing conducted on medical devices?
Biological safety testing on medical devices focuses on ensuring they are biocompatible. It includes cytotoxicity tests to check if the device harms cells, and irritation tests to assess whether it causes tissue damage. Sensitization testing determines if the device causes allergic reactions, while implantation tests check how devices interact with tissues over time. These tests ensure that medical devices, such as implants or surgical instruments, are safe for patient use and do not cause adverse health reactions.
7. How long does biological safety testing take?
The duration of biological safety testing can vary depending on the type of test. Simple tests like sterility may take a few days, while more complex tests like virus validation can take weeks or even months. Factors such as the complexity of the product, the number of tests required, and the type of biological material involved also influence the timeline. Manufacturers often have to balance the need for thorough testing with time constraints to meet product release schedules.
8. What are the challenges in biological safety testing?
Biological safety testing faces several challenges. One of the main issues is the high cost, as advanced testing methods and lab facilities can be expensive. The complexity of certain tests also makes them time-consuming, delaying product development. Regulatory compliance is another challenge, as testing must meet strict standards that vary by region. Furthermore, ensuring consistency in test results across different labs and systems is often difficult. These factors can increase the overall time and cost for companies conducting biological safety testing.
FAQs on the Biological Safety Testing Market
1. What is the size of the biological safety testing market?
The biological safety testing market is experiencing rapid growth. The Biological Safety Testing Market Size is anticipated to reach approximately USD 13.4 Billion by the year 2033, exhibiting a substantial growth from its valuation of USD 4.8 Billion in 2023. This growth is expected to transpire at a Compound Annual Growth Rate (CAGR) of 10.9% during the forecast period spanning from 2024 to 2033. This growth is driven by increased demand for biologics, medical devices, and pharmaceuticals. As regulatory standards become stricter, the need for biological safety testing rises. The market is also growing due to more clinical trials, drug development, and healthcare advancements. North America and Asia-Pacific are currently leading regions in this sector.
2. What are the key drivers of the biological safety testing market?
Key drivers of the biological safety testing market include increasing demand for biologics and cell-based therapies. The need for stricter safety regulations in the pharmaceutical industry also contributes to market growth. Additionally, the rising number of clinical trials and drug development activities, especially for novel therapies, fuels demand. Companies in the pharmaceutical and biotechnology sectors are focusing more on product safety, contributing to the market’s expansion. Growing awareness among consumers about product safety further drives the adoption of testing services.
3. What are the major challenges faced by the biological safety testing market?
The biological safety testing market faces several challenges, including high testing costs and the need for skilled professionals. Many tests require specialized equipment and expertise, making them expensive for companies. Stringent regulatory requirements also add complexity to the testing process. Furthermore, some traditional testing methods have technological limitations, which require continuous innovation. Ensuring compliance with changing regulations across different regions can also slow down testing and increase costs. These factors challenge market growth and accessibility for many organizations.
4. Which regions are witnessing the highest growth in the biological safety testing market?
North America leads the biological safety testing market due to its advanced healthcare infrastructure and regulatory framework. The region’s strong pharmaceutical and biotechnology industries drive demand for safety testing. However, the Asia-Pacific region is also witnessing significant growth. Increasing investments in biotechnology, improving healthcare systems, and growing awareness of safety standards contribute to this growth. Countries like China and India are expanding their pharmaceutical and medical device sectors, boosting the demand for biological safety testing in the region.
5. Who are the key players in the biological safety testing market?
Key players in the biological safety testing market include Charles River Laboratories, BSL Bioservice, Merck KGaA (MilliporeSigma), Samsung Biologics, Sartorius AG, Eurofins Scientific, SGS Société Générale de Surveillance SA, Thermo Fisher Scientific Inc., BIOMÉRIEUX, Lonza, and Other Key Players. These companies offer a range of testing services, including sterility, cytotoxicity, and virus validation. They provide crucial services to the pharmaceutical, biotechnology, and medical device industries. These players are leaders in the market due to their expertise, global presence, and extensive testing capabilities, helping ensure the safety of various biological products.
6. What are the emerging trends in the biological safety testing market?
Emerging trends in the biological safety testing market include the adoption of automation and digital tools to enhance testing accuracy and efficiency. These technologies reduce human error and speed up testing processes. Additionally, there is a growing focus on outsourcing biological safety testing to contract research organizations (CROs), enabling companies to reduce costs and improve efficiency. Another trend is the increased use of in-vitro testing methods, which reduce the reliance on animal testing. Personalized medicine is also leading to more targeted testing solutions.
7. What is the market outlook for biological safety testing?
The market outlook for biological safety testing is positive. The increasing demand for biologics and medical devices will continue to drive growth. With the rise in clinical trials and the focus on patient safety, biological safety testing will play an essential role in regulatory approvals. As regulatory standards become more stringent globally, the need for safety testing services will grow. Technological advancements in testing methods will also support market expansion. Overall, the market is expected to see steady growth in the coming years.
Conclusion
In conclusion, the biological safety testing market is set for significant growth, driven by increasing regulatory requirements, technological advancements, and the rising demand for biologics and medical devices. Stringent safety standards, along with innovations like in-vitro assays and AI-enhanced testing, are improving the efficiency and accuracy of safety assessments. The biopharmaceutical sector’s expansion and the global increase in healthcare infrastructure further support this growth. However, challenges such as high testing costs and regional regulatory complexities remain. Despite these hurdles, the market’s future outlook is promising, with substantial opportunities for companies offering compliant and innovative testing solutions.
View More
Sharps Safety Market || Infection Control and Biosafety Products Market || Cardiac Safety Services Market || Retractable Needle Safety Syringes Market || Environmental Health and Safety Software Market || Biological Safety Testing Products and Services Market || Pharmacovigilance and Drug Safety Software Market || ADME-Toxicology Testing Market || Genetic Toxicology Testing Market || Automated Cell Culture Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



