Introduction
The Biological Safety Testing Market is projected to expand from USD 4.8 billion in 2023 to approximately USD 13.4 billion by 2033, growing at a Compound Annual Growth Rate (CAGR) of 10.9% from 2024 to 2033. This significant growth is driven primarily by the increasing development within the biopharmaceutical industry, necessitating rigorous biological safety testing to ensure product safety and efficacy for regulatory approval.
Technological advancements are revolutionizing biological safety testing methods. Innovations in molecular and cellular biology have improved pathogen and contaminant detection, enabling more precise and rapid testing outcomes. These technological improvements are essential in adapting to the evolving needs of safety testing protocols.
Regulatory requirements also play a crucial role in shaping the market, with stringent guidelines ensuring that biological products, including vaccines and biologics, are free from contaminants and safe for human use. Additionally, the recent global health concerns have emphasized the importance of robust biological safety testing, driving increased investment in health safety measures.
The sector also benefits from efforts to enhance workforce development and workplace safety standards, crucial for maintaining high-quality testing environments. These efforts ensure the reliability and accuracy of testing processes, which are pivotal in meeting the strict regulatory standards and coping with the rapid advancements in biopharmaceuticals. The ongoing emphasis on professional training and safety standards underscores the dynamic nature of the biological safety testing market.
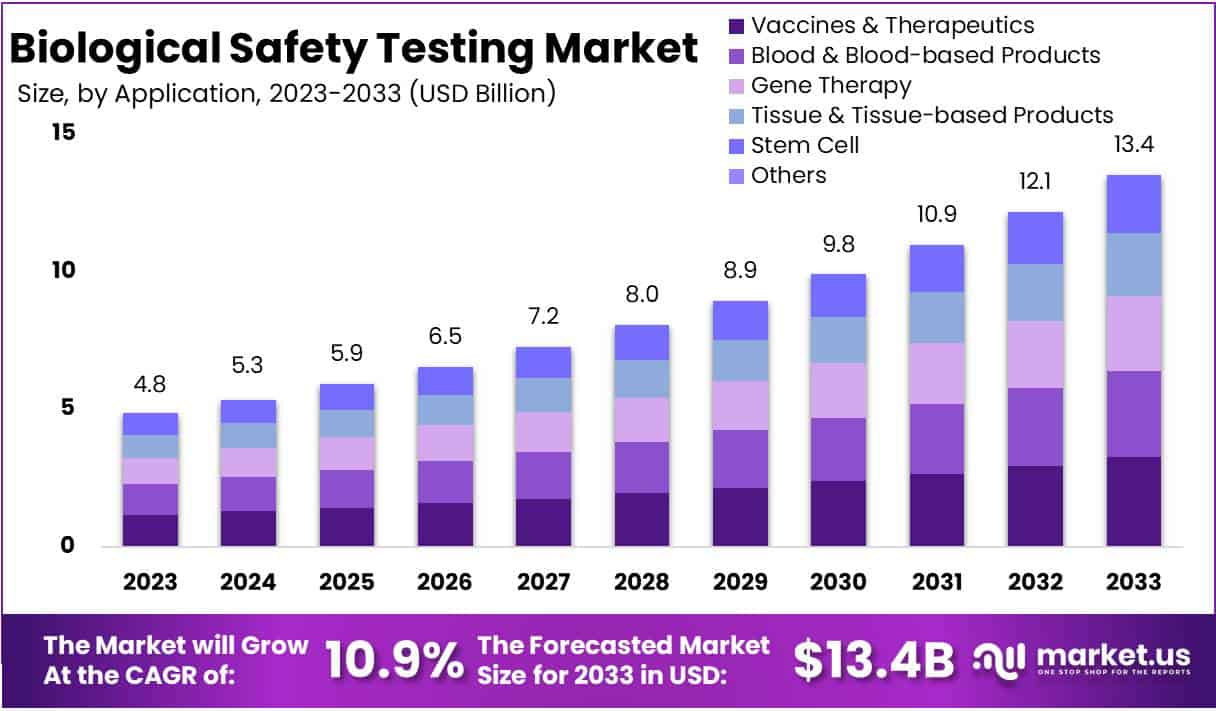
Key Takeaways
- The Biological Safety Testing Market is set to hit USD 13.4 billion by 2033, growing at a steady CAGR of 10.9% from 2024.
- Reagents & Kits led product categories in 2023, holding 39.7% of the market, essential for precise biological safety testing.
- Vaccines & Therapeutics were prominent in application, taking up 24.1% of the market in 2023, highlighting their importance in safety testing.
- Endotoxin Tests were the most used test type in 2023, with a 22.6% share, crucial for detecting harmful toxins in products.
- Increased investment in biologics and biosimilars research and development is a major driver, boosting the market’s growth.
- The expanding pharmaceutical and biotechnology sectors are increasing the demand for biological safety testing to ensure product safety.
- High costs of testing are a significant barrier, especially for smaller firms or those with fewer resources.
- There’s a rising opportunity in the outsourcing of biological safety testing services, meeting the increasing market demands.
- Technological progress, like the use of 3D tissue models, is enhancing the effectiveness of biological safety tests.
- North America dominated the market in 2023 with over 36.2% share, supported by its strong healthcare system and strict regulations.
Emerging Trends
- Artificial Intelligence in Biosecurity: The integration of AI into biological safety testing is expanding, enhancing the speed and accuracy of identifying pathogens and toxins. AI technologies are being explored to predict and mitigate biological risks before they manifest, especially in high-risk research scenarios.
- Advanced Diagnostic Playbooks: To combat biological emergencies, there’s a movement toward developing comprehensive diagnostic playbooks. These guides aim to standardize rapid and effective testing protocols during outbreaks, ensuring that responses are swift and coordinated across various health sectors.
- Reduction of Animal Testing: Legislative and technological advancements are leading to significant reductions in animal testing. This is achieved through the development of alternative methods that provide more humane and scientifically valid safety data for drugs and other products.
- Increased Biodefense Preparedness: Programs like the Apollo Program for Biodefense aim to end the era of pandemics by enhancing U.S. and global preparedness through advanced technologies. This includes improving detection systems and accelerating vaccine development to respond more effectively to biological threats.
- Biosecurity Leadership Training: There’s a growing emphasis on training the next generation of biosecurity leaders. Programs are being established to equip professionals with the necessary skills and knowledge to handle and mitigate biological threats effectively.
Use Cases
- AI-Driven Threat Identification: AI tools are being utilized to analyze vast data sets to identify potential biological threats quickly. This helps in the timely implementation of containment and mitigation strategies, especially in densely populated areas or high-risk environments.
- Rapid Testing Deployment: In response to the COVID-19 pandemic, new frameworks for deploying diagnostic tests rapidly have been developed. These frameworks are designed to be adaptable to various types of biological emergencies, ensuring that testing is accessible and equitable.
- Non-Animal Testing Technologies: Innovations in biosafety testing are increasingly relying on non-animal methods, such as in vitro systems and computer models, to evaluate the safety and efficacy of products. This shift not only reduces the ethical concerns associated with animal testing but also often improves the speed and accuracy of results.
- National and Global Biodefense Strategies: The development of national strategies to enhance biodefense capabilities reflects a proactive approach to managing biological threats. These strategies focus on integrating cutting-edge technologies and fostering international collaboration to enhance global response capabilities.
- Educational Programs for Biosecurity: Specialized educational programs are preparing professionals to tackle current and future biological risks by providing them with in-depth training on the latest biosecurity and biodefense techniques. These programs are crucial for building a robust workforce capable of responding to global health security challenges.
Regional Analysis
North America secured a leading position in the Biological Safety Testing Market in 2023, boasting a market share of 36.2% and a value of USD 1.73 billion. The region benefits from an advanced healthcare infrastructure and rigorous regulations enforced by authorities like the FDA and Health Canada. These regulations ensure the safety of pharmaceuticals and biologics, bolstering market confidence.
The demand for biological safety testing in North America is propelled by its dynamic biopharmaceutical sector. Companies in the region are deeply engaged in pioneering research, which necessitates thorough safety evaluations. The emphasis on technological innovation and research excellence positions North America at the forefront of developing cutting-edge safety testing methods.
Challenges such as the COVID-19 pandemic have underscored the importance of stringent safety testing. North America’s proactive approach in handling public health crises and its investments in diagnostic solutions further amplify the need for comprehensive safety protocols. This ensures ongoing enhancements in testing standards, pivotal for addressing current and future health emergencies.
Looking forward, North America is poised to maintain its dominance in the Biological Safety Testing Market. With robust regulatory frameworks, a vibrant biopharmaceutical industry, and a commitment to technological advancements and public health, the region is well-equipped to capitalize on emerging opportunities and steer the future of biological safety testing.
Conclusion
The Biological Safety Testing Market is set to undergo significant growth, driven by advancements in biopharmaceutical development and an increasing need for rigorous safety protocols. As the market evolves, the integration of cutting-edge technologies and stringent regulatory frameworks will further bolster the demand for effective safety testing. The commitment to reducing animal testing and enhancing biosecurity measures highlights the industry’s adaptability and readiness to address emerging global health challenges. With a focus on technological innovation and workforce development, the market is well-positioned to meet the expanding requirements of biological safety, ensuring products are both safe and effective for consumer use. This market’s progression reflects an industry adapting swiftly to the complex demands of healthcare and regulatory standards.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



