Table of Contents
Introduction
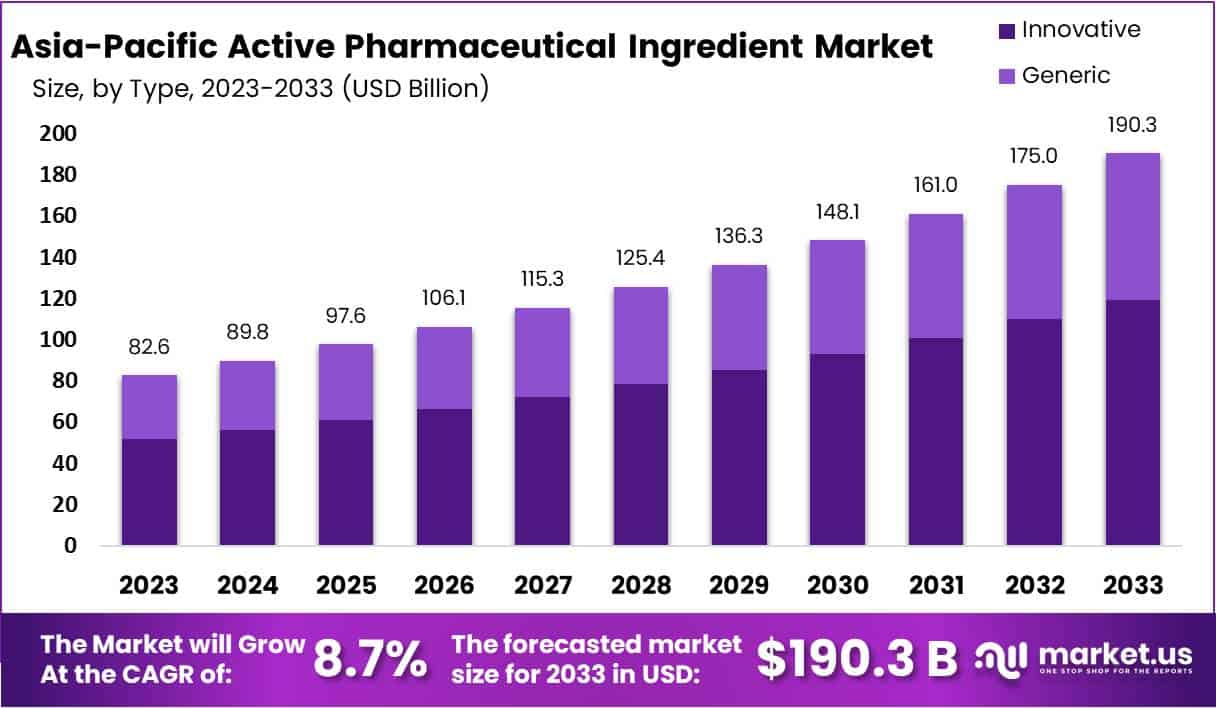
The Asia-Pacific Active Pharmaceutical Ingredient (API) market is projected to reach approximately USD 190.3 billion by 2033, rising from USD 82.6 billion in 2023. This growth is expected at a Compound Annual Growth Rate (CAGR) of 8.7% from 2024 to 2033. Several factors are driving this expansion, including local production initiatives, clinical trial advancements, regulatory support, and digital transformation in the pharmaceutical sector.
One key factor contributing to this growth is the increasing focus on local API production. Governments across the region have introduced policies to encourage domestic manufacturing. For instance, Pakistan launched an API promotion policy in 2022, resulting in enhanced production capacity and the establishment of new API manufacturing facilities. These efforts aim to improve health security by reducing dependency on imported pharmaceutical ingredients.
The expansion of clinical trials has also significantly impacted the API market. The region is experiencing a steady rise in clinical trial activities, with an anticipated CAGR of 6.7% in this segment. This growth reflects the increasing demand for early-phase clinical trials, positioning the Asia-Pacific region as a key hub for pharmaceutical research and development. The rising number of clinical studies has further boosted the demand for APIs used in investigational treatments and drug development.
Regulatory support for biosimilar drugs is another notable factor driving the market. Countries such as Japan, South Korea, and Malaysia have established clear guidelines to promote the development and approval of biosimilars. These regulations encourage pharmaceutical companies to produce biosimilar products, expanding the API market by facilitating the entry of cost-effective alternatives to biologic drugs.
Digital transformation has further contributed to market growth. The integration of digital platforms and the rise of online pharmacies have expanded access to pharmaceutical products, including APIs. This shift has improved supply chain efficiency and streamlined distribution processes, enhancing market penetration across the region. As digital adoption continues to grow, the API market is expected to benefit from improved accessibility and increased demand for pharmaceutical ingredients.
Collectively, these factors highlight the dynamic landscape of the Asia-Pacific API market. The combination of local production incentives, clinical trial growth, regulatory advancements, and digital transformation has created a favorable environment for market expansion. As these trends continue to evolve, the region’s pharmaceutical sector is poised for sustained growth in the coming years.
Key Takeaways
- Market Growth: The Asia-Pacific API market is projected to reach USD 190.3 billion by 2033, growing at a CAGR of 8.7% from 2024.
- Innovative Segment: Innovative APIs dominate with a 62.7% share, supported by rising R&D investments and favorable government policies promoting innovation.
- Captive Segment: The captive API segment holds a 61.5% share, benefiting from improved quality control through in-house production by major pharmaceutical companies.
- Synthetic APIs: Synthetic APIs lead with a 76.2% share, preferred for their accessibility, stable supply chains, and cost-effective manufacturing processes.
- Clinical Applications: APIs for clinical applications account for 71.3% market share, reflecting growing demand for effective pharmaceutical treatments.
- Cardiology Segment: Cardiology APIs dominate with a 21.4% market share, driven by increasing cases of cardiovascular diseases worldwide.
- China’s Market Share: China holds a 41.2% market share, valued at USD 34 billion in 2023, backed by its strong production capacity.
- India’s Growth Potential: India is poised for growth due to its efficient manufacturing processes and expanding presence in the generic drugs market.
- Outsourcing Opportunities: Outsourcing API manufacturing is gaining traction, offering pharmaceutical companies cost savings and access to specialized production capabilities.
Emerging Trends
- Shift Towards Self-Reliance in API Production: Countries in the Asia-Pacific region are moving toward self-sufficiency in API manufacturing. India, for instance, has launched initiatives to reduce its reliance on imports. These efforts focus on strengthening domestic API production. This strategic move aims to improve supply chain resilience. By enhancing local manufacturing capabilities, the region can ensure a steady supply of essential medicines. This shift is crucial to prevent disruptions in healthcare services and promote national health security.
- Expansion of Biosimilar APIs: The demand for biosimilar APIs is rising in the Asia-Pacific region. In 2024, Glenmark Pharmaceuticals introduced a biosimilar version of Liraglutide, an anti-diabetic drug. This launch marked Glenmark’s entry into the injectable anti-diabetic therapies sector. As biosimilars provide cost-effective treatment options, their growing presence is transforming the healthcare landscape. Such innovations are vital in improving patient access to affordable medications.
- Development of Generic Weight-Loss APIs: Pharmaceutical companies in Asia-Pacific are increasingly developing generic weight-loss drugs. Indian firms are preparing to launch generic versions of Saxenda, a popular weight-loss medication. Biocon plans to introduce its version of Saxenda in the UK by November 2024. The company expects annual sales to reach £18 million following the drug’s patent expiry. Biocon is also seeking EU approval this year and US approval by 2025. This growth reflects rising demand for affordable weight-loss treatments.
- Integration of Advanced Manufacturing Technologies: API manufacturers in Asia-Pacific are adopting advanced manufacturing technologies. Continuous manufacturing processes and automation are gaining popularity. These innovations improve production efficiency and product quality. By enhancing scalability, manufacturers can meet growing demand more effectively. Additionally, automated systems reduce operational costs and minimize human error. This shift is driving greater competitiveness in the API sector.
- Strengthening Regulatory Compliance: Companies in the Asia-Pacific region are strengthening regulatory compliance efforts. Investments are being made to upgrade manufacturing facilities and processes. These improvements align with international Good Manufacturing Practices (GMP) standards. By meeting stringent regulatory requirements, companies can expand their presence in global markets. Compliance with these standards ensures product safety, efficacy, and quality. This approach supports stronger partnerships with international pharmaceutical firms.
Use Cases
- Antimalarial Treatments: Artemisinin is a key API used in antimalarial therapies. This compound is derived from the sweet wormwood plant. China and Vietnam are major suppliers, contributing around 70% of the global raw material for artemisinin production. The price of artemisinin has seen significant fluctuations, ranging from US$120 to $1,200 per kilogram between 2005 and 2008. Due to the Asia-Pacific region’s strong agricultural presence, the supply chain for artemisinin remains stable. This ensures consistent production of effective antimalarial treatments, supporting both regional and global healthcare needs.
- Generic Drug Manufacturing: The Asia-Pacific region, especially India, is a prominent player in generic drug manufacturing. Companies like Ipca Laboratories produce over 150 pharmaceutical formulations. These include oral liquids, tablets, dry powders, and capsules. These generic drugs are widely distributed in both domestic and international markets. India’s cost-effective production methods and skilled workforce have positioned the region as a global hub for affordable medications. This has improved access to essential treatments in developing countries.
- Development of Biosimilars: The Asia-Pacific region is advancing rapidly in biosimilar development. Biosimilars provide cost-effective alternatives to expensive biologic therapies. Glenmark Pharmaceuticals introduced a biosimilar version of the anti-diabetic drug Liraglutide in 2024. This launch marked a significant achievement for the Indian pharmaceutical sector. Biosimilars improve treatment accessibility for patients requiring long-term therapies. Their affordability has the potential to reduce healthcare costs and expand access to vital medications across the region.
- Production of Weight-Loss Medications: Pharmaceutical firms in the Asia-Pacific region are investing heavily in weight-loss medication development. Sun Pharmaceuticals, India’s largest drug manufacturer by revenue, is set to launch an experimental anti-obesity and type 2 diabetes drug within five years. Known as Utreglutide (GL0034), this medication is a GLP-1 receptor agonist. It mimics gut hormones, helping to reduce appetite and improve blood sugar levels. This innovation could offer significant benefits for managing obesity and diabetes, supporting public health efforts in the region.
Conclusion
The Asia-Pacific Active Pharmaceutical Ingredient (API) market is poised for significant growth, driven by key factors such as increasing local production, clinical trial advancements, regulatory support, and digital transformation. Efforts to promote domestic manufacturing have improved supply chain stability, reducing dependency on imports. The expansion of biosimilars, weight-loss medications, and innovative APIs has further boosted market growth. Additionally, advancements in manufacturing technologies and stronger regulatory compliance are enhancing product quality and production efficiency. With a growing focus on affordable treatments and improved healthcare access, the API market in the Asia-Pacific region is expected to witness sustained expansion, providing opportunities for pharmaceutical companies and healthcare providers alike.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



